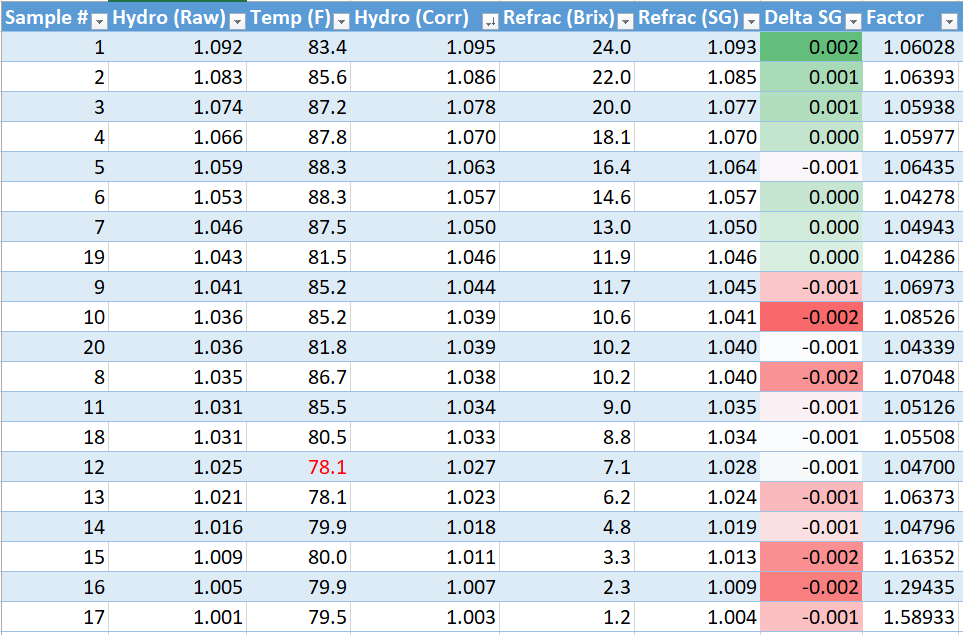
We're always told not to trust the specific gravity scale in a refractometer, and that you need to calibrate it by referring to a hydrometer. Having too much time on my hands currently, I made a solution with DME and did a bunch of dilutions and measurements. Here are the results.
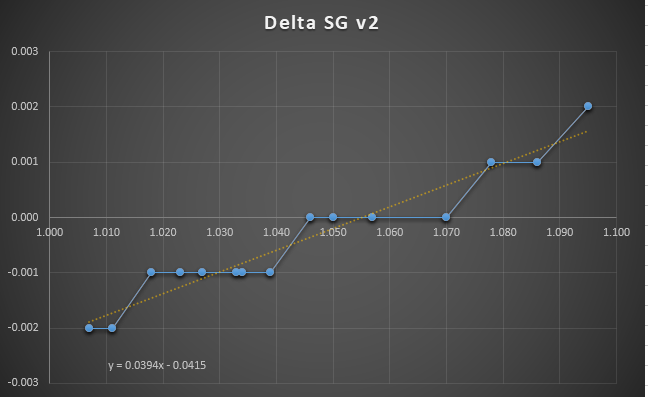
The chart is sorted by the "authoritative" value, the temperature corrected hydrometer reading in SG. The colored column shows the delta between that value and the refractometer SG reading. I also captured the refractometer brix reading.
A few values were discongruous, for example sample #5 showed a difference of 1 unit though the surrounding values were all 0. In some of these cases, I took new readings, and the re-tests did seem to be more in line with the trend.
If you eliminate the outliers, you can make a chart like this:
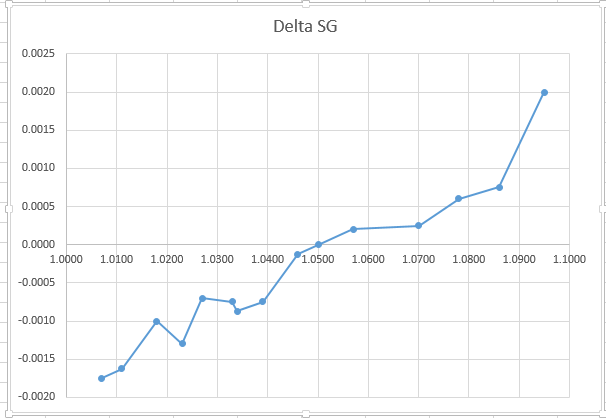
It seems like you can fit a somewhat linear curve to the data in the beginning, but as gravity increases, you get into a non-linear correction.
From approximately 45 to 75 gravity units, the indicated correction is less than one-half a unit, meaning that I guess I could basically trust the SG value on the instrument if it falls in that range. Below ~45 units I should add 1 point to the indicated value; above 75 units, I should subtract one, and above about 90 units, I should subtract 2.
I guess the good news is, for my instrument, if I just trust it I am unlikely to be off by more than 2 units, and likely just 0-1. That isn't bad, I am not launching a rocket.
BUT, I don't yet understand how different kinds of wort might change these values. The test solutions were made with light DME only. If worts of different origins don't follow this kind of curve, then the value of a refractometer seems dubious.
The data also shows a "wort correction factor" as calculated on the Brewer's Friend spreadsheet. The last couple of values, at very low gravities, are way out of line with the rest, which makes me suspicious. If you eliminate any value over ~1.10, the average correction factor is 1.054. If we used that as the authoritative correction across the whole range, the correction results are similar, but the breakpoints are a little different.
Bottom line, I am more confused than when I started. I might go back to my hydrometers for pre-fermentation readings!
I might go back to my hydrometers for pre-fermentation readings!

The chart is sorted by the "authoritative" value, the temperature corrected hydrometer reading in SG. The colored column shows the delta between that value and the refractometer SG reading. I also captured the refractometer brix reading.
A few values were discongruous, for example sample #5 showed a difference of 1 unit though the surrounding values were all 0. In some of these cases, I took new readings, and the re-tests did seem to be more in line with the trend.
If you eliminate the outliers, you can make a chart like this:

It seems like you can fit a somewhat linear curve to the data in the beginning, but as gravity increases, you get into a non-linear correction.
From approximately 45 to 75 gravity units, the indicated correction is less than one-half a unit, meaning that I guess I could basically trust the SG value on the instrument if it falls in that range. Below ~45 units I should add 1 point to the indicated value; above 75 units, I should subtract one, and above about 90 units, I should subtract 2.
I guess the good news is, for my instrument, if I just trust it I am unlikely to be off by more than 2 units, and likely just 0-1. That isn't bad, I am not launching a rocket.
BUT, I don't yet understand how different kinds of wort might change these values. The test solutions were made with light DME only. If worts of different origins don't follow this kind of curve, then the value of a refractometer seems dubious.
The data also shows a "wort correction factor" as calculated on the Brewer's Friend spreadsheet. The last couple of values, at very low gravities, are way out of line with the rest, which makes me suspicious. If you eliminate any value over ~1.10, the average correction factor is 1.054. If we used that as the authoritative correction across the whole range, the correction results are similar, but the breakpoints are a little different.
Bottom line, I am more confused than when I started.









































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)