How should the typical formula for DME addition to starter wort deal with the volume contribution of the DME itself?
Assume a PPG for the DME is 42 points/lb/gal.
To make 1 gallon of wort with SG=1.042, I would (per common practice) mix 1 gallon water with 1 lb of DME. Please ignore the high SG; I know it should be 1.030-1.040.
According to Pierce, 1 lb of DME adds 9.42oz of volume.
So, making the above mixture creates 1gal+9.42oz = 1.07359375 gal.
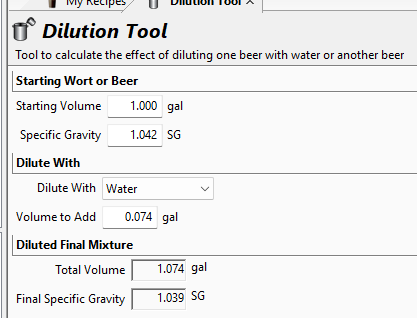
If I use the volume of the DME as a "dilution", the SG changes (ever so slightly; see screenshot below):
Should I be thinking about the total points in the contents of the solution that I have? If that's the case, then this 1.074 gallons of solution has contained within it 42 points worth of sugar.
Would this imply that 1.000 gallon of this solution would contain (1.000 / 1.074) * 42 = 39 points worth of sugar?
If so, then the SG for the starter wort is 1.039, not 1.042. (I won't even try to add the complication of adding the volume of the yeast slurry )
)
Your thoughts will be very much appreciated.
References:
https://byo.com/article/extract-for-all-grainers-advanced-brewing/
https://www.homebrewtalk.com/threads/question-about-ppg-definition.729503/
Assume a PPG for the DME is 42 points/lb/gal.
To make 1 gallon of wort with SG=1.042, I would (per common practice) mix 1 gallon water with 1 lb of DME. Please ignore the high SG; I know it should be 1.030-1.040.
According to Pierce, 1 lb of DME adds 9.42oz of volume.
So, making the above mixture creates 1gal+9.42oz = 1.07359375 gal.
If I use the volume of the DME as a "dilution", the SG changes (ever so slightly; see screenshot below):
Should I be thinking about the total points in the contents of the solution that I have? If that's the case, then this 1.074 gallons of solution has contained within it 42 points worth of sugar.
Would this imply that 1.000 gallon of this solution would contain (1.000 / 1.074) * 42 = 39 points worth of sugar?
If so, then the SG for the starter wort is 1.039, not 1.042. (I won't even try to add the complication of adding the volume of the yeast slurry
Your thoughts will be very much appreciated.

References:
https://byo.com/article/extract-for-all-grainers-advanced-brewing/
https://www.homebrewtalk.com/threads/question-about-ppg-definition.729503/







































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)


















