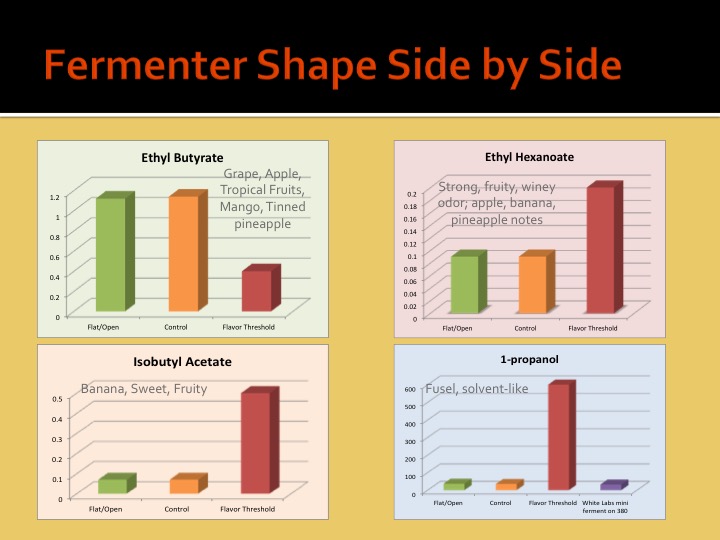
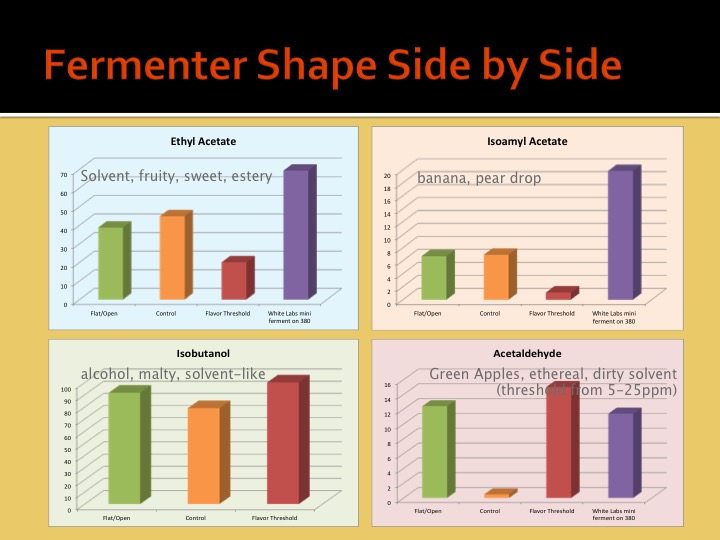
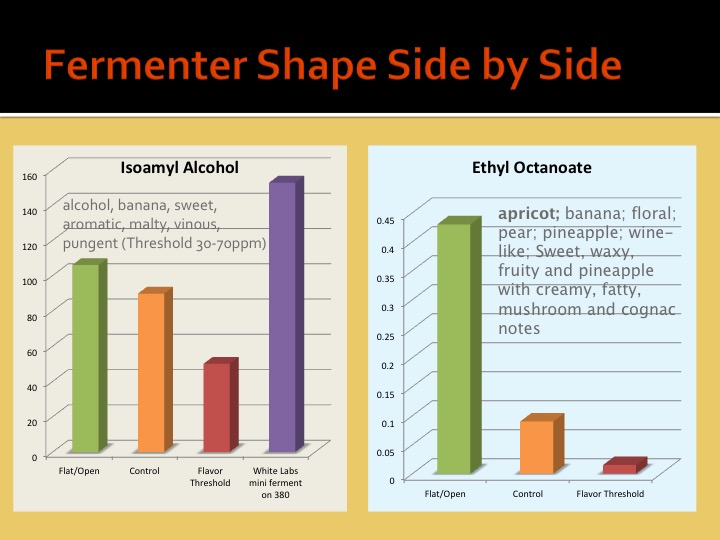
What effects are you specifically talking about and why are you assuming the effects of geometry are proportional (aside from pressure)?
The depth of liquid (and static pressure) in homebrew sized vessels is less than the shallowest of commercial tanks. On the commercial scale the difference in pressure between a cylindroconical vessel and a shallow fermentor are significant. Not so when you're talking about 8" difference in liquid level between a carboy and a plastic tub.
Stick with me, here.
In response to the pressure part:
If we assume that a characteristic of fluid mechanics is responsible for ester increase, then that characteristic would be pressure. Since the pressure goes down in a rectangular prism fermenter, then the ester production goes up when the pressure is less, via our first assumption.
If that was true, then the difference in pressures between commercial brewing and home brewing would be immense and homebrewers would notice
increased ester production (relative to style) in
every beer we made as opposed to commercial.
This is why I do not believe that increased ester production is a function of pressure.
In response to the geometry part:
We can look at proportionality between any characteristic of a 3D shape and it's volume. It will be a constant value.
Say you have a cubic fermenter that holds some volume "V" of wort and has a base area "A" at the bottom. You can compare, for instance, the area of the base of the fermenter with the volume it caries:
A/V=C
Where "C" is your base area to volume ratio. If you wanted to make a fermenter that holds more volume "V" and maintains the same base area per volume ratio, then you can construct a fermenter with an equal base area per volume:
C*V=A,
Where "A" is the product of the lengths of two adjacent sides of your rectangular base.
The point of this is, in short, that we keep the attributes of what the beer is contacting the same. Two fermenters constructed proportionally in this manor will sit on the same area of flocculated yeast per unit volume of beer. Or it will be in touch with the same area of surface area to air per unit volume.
We must be able to surmise that ester production is proportional to some characteristic of the shape of the fermenter, and not the pressure of the fluid within.
In other words, pressure is the only property at work here for which scale could account for differences. Since pressure is not a likely suspect, then it only leaves the actual shape of the fermenter. Since the shape of a fermenter can be proportional for any unit volume, scale does not matter.





























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)