roblanderson
Well-Known Member
- Joined
- Mar 17, 2013
- Messages
- 49
- Reaction score
- 3
I spoke with someone at our city water department and she sent me the numbers I need to enter into Bru n water. See the attached screenshot for the details.
When I enter this into the Bru'n Water spreadsheet I'm told that the Cation/Anion difference is showing an unbalanced report. They should be within 0.5 of each other and instead it is 4.84 - quite a difference.
Have I misinterpreted the numbers I was given? Do they need to be converted first maybe?
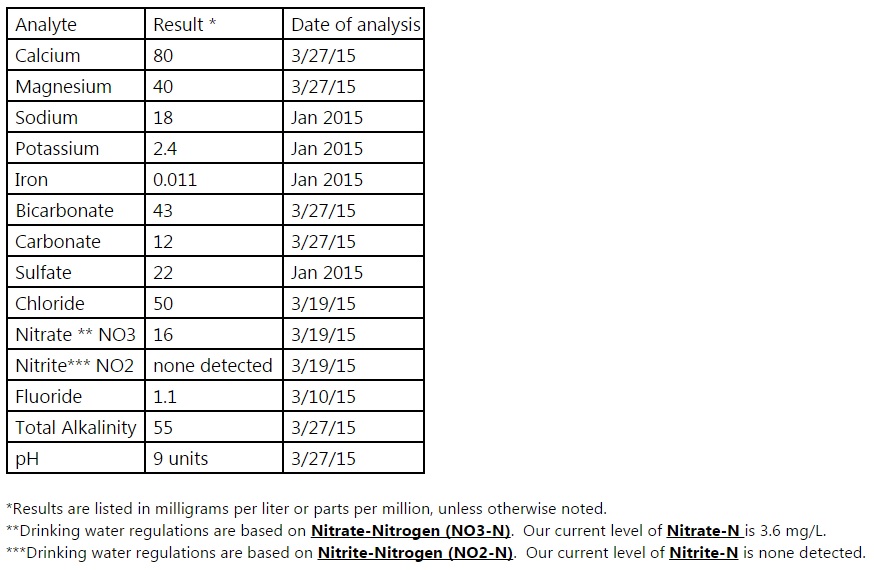
When I enter this into the Bru'n Water spreadsheet I'm told that the Cation/Anion difference is showing an unbalanced report. They should be within 0.5 of each other and instead it is 4.84 - quite a difference.
Have I misinterpreted the numbers I was given? Do they need to be converted first maybe?
























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)