Ok, I made some assumptions (e.g. grain wort absorption and boil off rate, 5 gallon batch) which may not match your own, but assuming you're mashing with approx. 7.9 gallon of water, here's what I would probably do.
In Mash: 2.5 grams CaCl2 (just to get a bit of calcium in the mash as an enzyme cofactor and alkalinity offsetter, and some chloride for rounded malty-ness). Mash pH should land in the vicinity of 5.5.
Add After Mash:
1.5 grams CaCl2 (calcium for yeast flocculation, Cl2 for rounded malty-ness)
2 grams CaSO4 (more calcium for yeast flocculation, and a bit of sulfate to balance all the chloride a little)
5.5 grams NaCl (Sodium enhances the chocolate-y flavors)
ETA: the above will result in a profile similar to my big stouts.
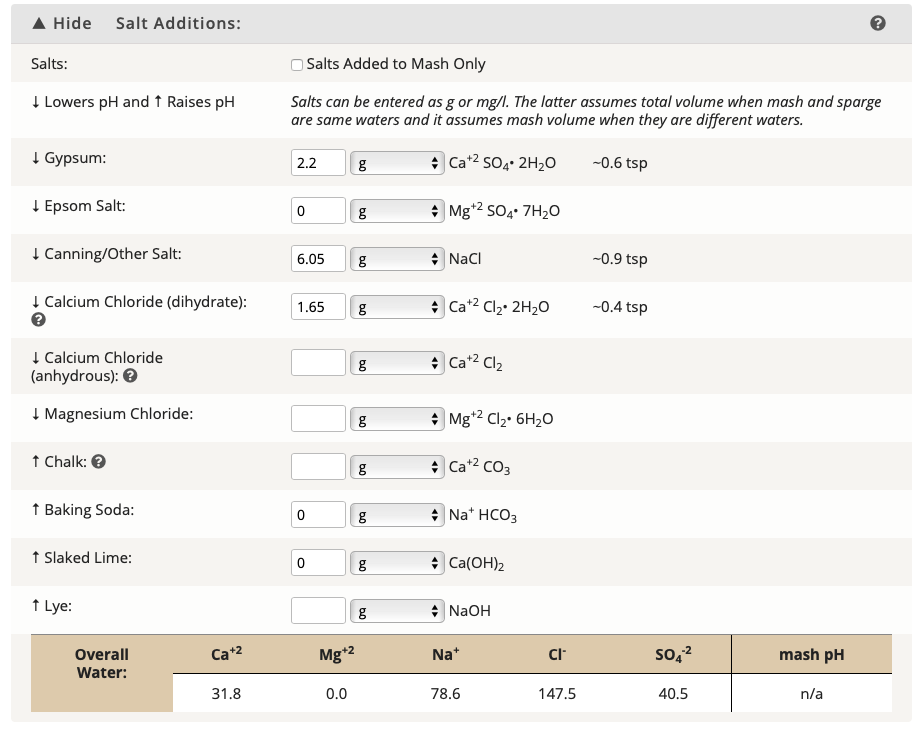
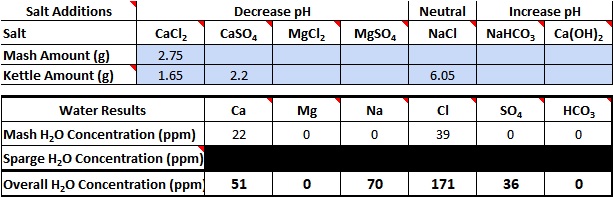
So..I threw these numbers into BrewersFriend, and this is the result. Is this accurate?
I thought you wanted your Ca and Sulfate numbers to be at least 50? Or is the Sulfate number just on the cusp what is generally accepted as a 2:1 ratio and you stretched it to 3:1?



















































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)








