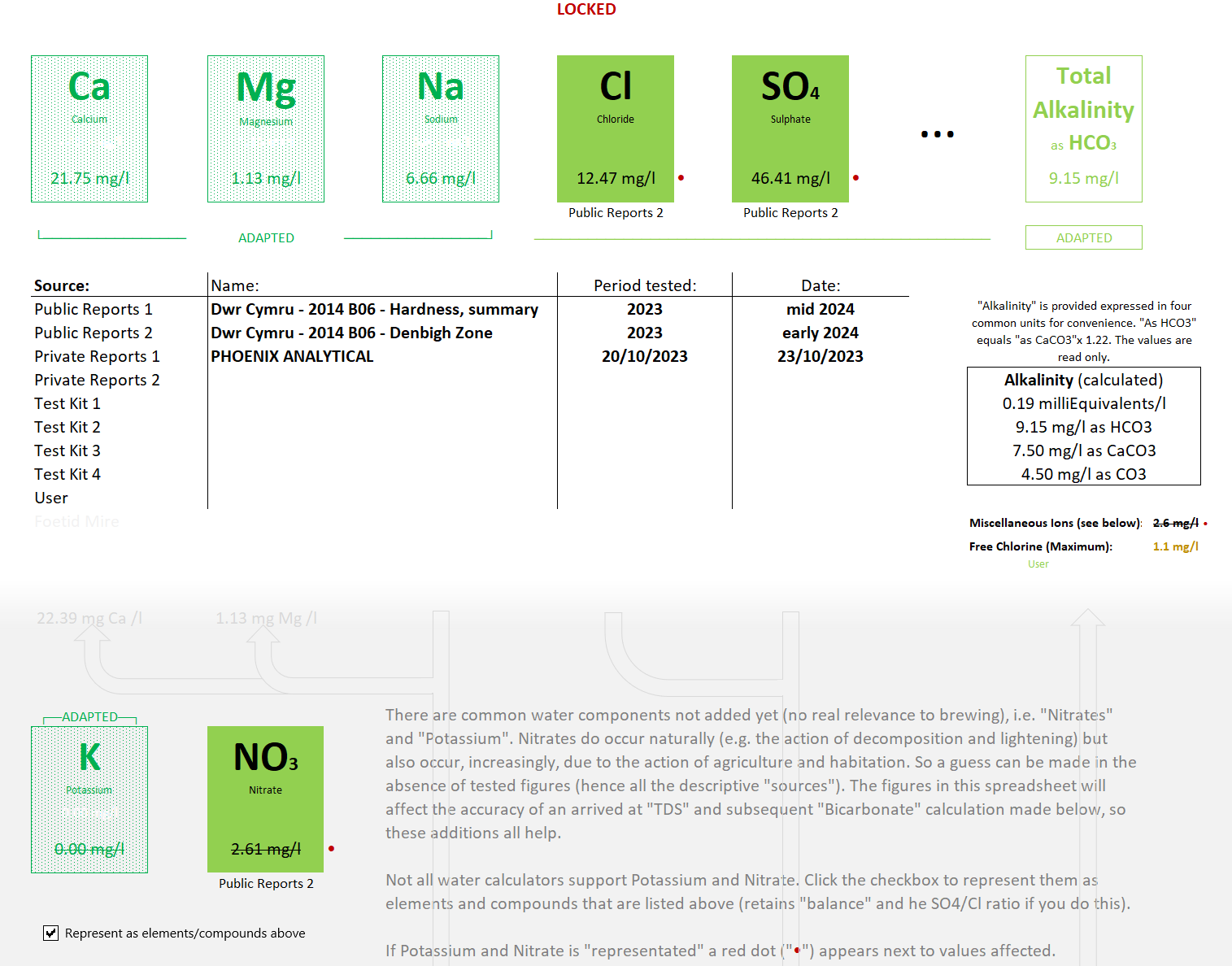
I was doing something with that wretched "Defuddler" spreadsheet of mine, and I remembered this thread and
@mac_1103's frustrations with it, so I thought I'd chuck this piece in:
@mac_1103 I understood alkalinity to be a measurement rather than a considered value. Based on the formula for total alkalinity ≈
50.0435(Ca/20.039 + Mg/12.153 + Na/22.99 - Cl/35.453 - SO4/48.031).
My results are 219ish, based off of using a quantity of "0" for and SO4.
Whoa! What a lot of complicated screwy numbers! That's got to be more correct than I'm saying?
Except ... not at all! It's doing the same thing. All the screwy numbers are changing things back-and-fro with "equivalence" measurements. I recognise some of the numbers; 20 and 50 (20.039 and 50.0435 wouldn't be used on the back of a fa... ah-ah

... cigarette-packet), so I can see "as CaCO
3" is involved. And "equivalence" means "the same". So, you can use simple arithmetic (plus and minus) with them. Even someone brain-damaged can do it (that's not being politically incorrect ...
I am brain-damaged!). Let me roll out my forever-malevolent (it's actually quite useful!) "Defuddler" spreadsheet:
So there's lots going on in that snippet of the spreadsheet, but the first line is much the same as the quote it follows. The same elements and compounds, the same "result" - "Alkalinity" - I've just displayed the resulting units ("mg/l" which is "ppm" here). The "equivalents" are worked in the depths of the spreadsheet, along with the dead-simple sums ... add the cations together ("1+2=3" fashion!), add all the anions, subtract the anions from the cations and what you're left with is remaining anions that represent "alkalinity", as near as damn-it.
My spreadsheet will incude things like the potassium and nitrates shown above, but that's just nit-picking as far as drinking water is concerned.
The "Alkalinity" is displayed in four different units, of which I have a preferrence (as do most of your water companies) for "as bicarbonate", which is reasonble as 95-97% of alkalinity
is bicarbonate (HCO
3) in drinking water.
And the purpose of this post? Just to prove that the often repeated addage, "water chemistry is complicated" is nonsense at this level. It's just those scary long numbers, the outlandish units (like "mEq/L"), and most of all the out-dated technologies like "Hardness" and "as CaCO
3" (even "mEq/L" that I use a lot is outdated amongst the big boys) that causes the confusions.
And why do I bother to write these loooong drawn out posts. I did mention I'm "brain-damaged" (stop it, horrid term, ... brain-injured). Well these long drawn out posts are one way I use to drive things home in me 'ead.


























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)
































 ... cigarette-packet), so I can see "as CaCO
... cigarette-packet), so I can see "as CaCO