You raise fair points!You may have just set a record for off-topic-edness.
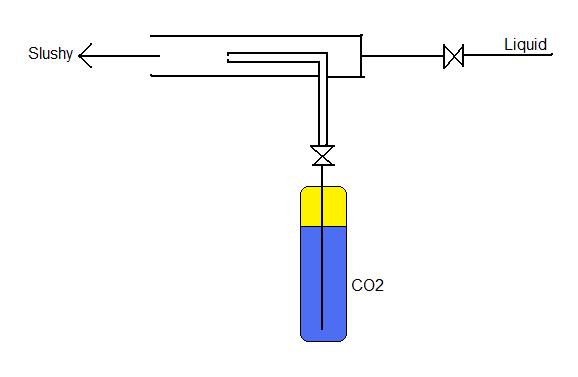
Still not totally clear here, but you could mix liquid CO2 with liquid sorbet under high pressure. At these high pressures, the CO2 can be at equilibrium as a liquid. Then supercool the liquid to solidify the sorbet. At this point, the CO2 is dissolved in the liquid. As it melts, the CO2 would come out of equilibrium and make the sorbet appear to boil. I am not totally sure if it will work, but it is sound in theory. (I do know the same process is used with plastic and when heated to a softening point, the plastic bubbles up. Unfortunately, sorbet isn't particularly rigid and may be near its softening point at freezer temps. You may have to store it in especially cold freezers...like Dippin' Dots! Probably couldn't sell it in the supermarket, though, because it would likely soften on the way home.)
Good luck in your endeavors and be careful.
Also, I was considering doing that with mixing them, but the only problem is that I would think that would use too much liquid CO2 at once. Go back 1-2 pages and read the MIT study on carbonated ice cream.






























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)