If you rely on a bottle of CO2 gas for carbing your kegged beer, there are basically two ways to go about it; set and forget and what I'll call "burst carbing". Some folks talk about this second method as "force carbing" but it's all done with force so forget it. For example sake, let's assume you want to carb your 45ºF beer to 2 volumes of CO2....
Set and forget relies on certain gas laws that will determine the carbonation level based on pressure and temperature. The volumes of carbonation will eventually reach an equilibrium to the head space pressure that is applied (what the regulator is set to). You'd use the charts like this one
http://www.kegerators.com/carbonation-table.php to figure out what that pressure needs to be. In our example, the chart shows it would take 9 psi to reach 2 volumes in your 45ºF beer.
The second method, burst carbonation, uses a much higher initial pressure and even some gas diffusion techniques like shaking or airstones to encourage a quicker solution of the gas. In our example, you might put 30psi on initially. If you refer back to the chart, you'll see this pressure, if left long enough will equilibrate to 3.79 volumes given enough time. The trick/difficulty in this method is knowing how long to leave it at the elevated pressure to get close to your desired volumes without overcarbing.
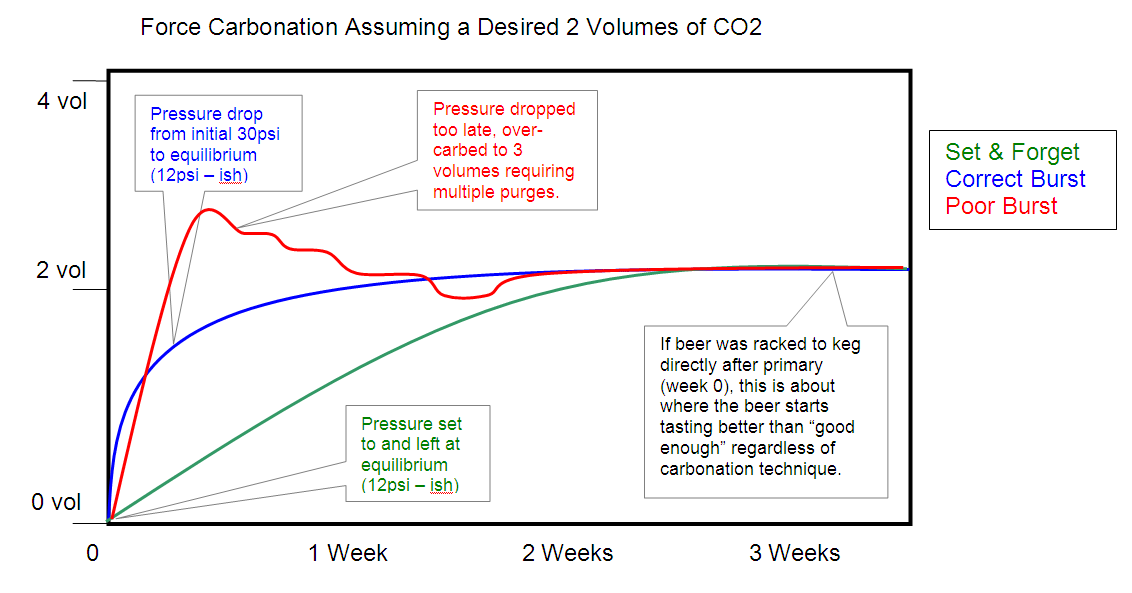
Some people understand pictures better than words so I drew this.
The green line is the set and forget method. You can see that it will take about 2 weeks to reach your desired volumes. Some folks will argue that they have carbonation in 1 week but "some" carbonation is not exactly equilibrated carb level though you might enjoy it anyway. I'm not 100% sure how long it takes but I have noted an increased carb level between week 1 and 2 on more than a few batches so I'm calling it 2 weeks to get it pretty close. You'll notice a small increase from week 2 to 3 but it's slight.
The blue line is just an example of a well executed boost carb. You'd leave it at approximately 3 times the equilibrium pressure for 24 hours, then drop it down and purge the keg so the headspace is now at the "chart pressure". If you do it right, you'll get close and then it will only take a couple more days to reach your desired volumes.
Highlighted for emphasis: More often than not, people in a hurry will try boosting even more by going with higher pressures and/or shaking the heck out of the keg. This usually results in what the red line is showing. You overshoot the carb level and then fight with the keg for several days to get it back down by purging the pressure a few times.
The final point I want to make is that the only reason I'd advocate a boost carb is when your beer has already aged/conditioned prior to making it to your kegerator and you need the beer to be drinkable in less than two weeks (poor planning on your part of course). I noted on the chart that if you went from primary right to keg at week zero, no matter how fast you carb, it will still take at least 3 weeks to taste decent. Therefore, why boost carb at all?









































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)

















 .
.  good to know.
good to know.