Lakeside_Brewer5
Member
- Joined
- Sep 18, 2020
- Messages
- 24
- Reaction score
- 14
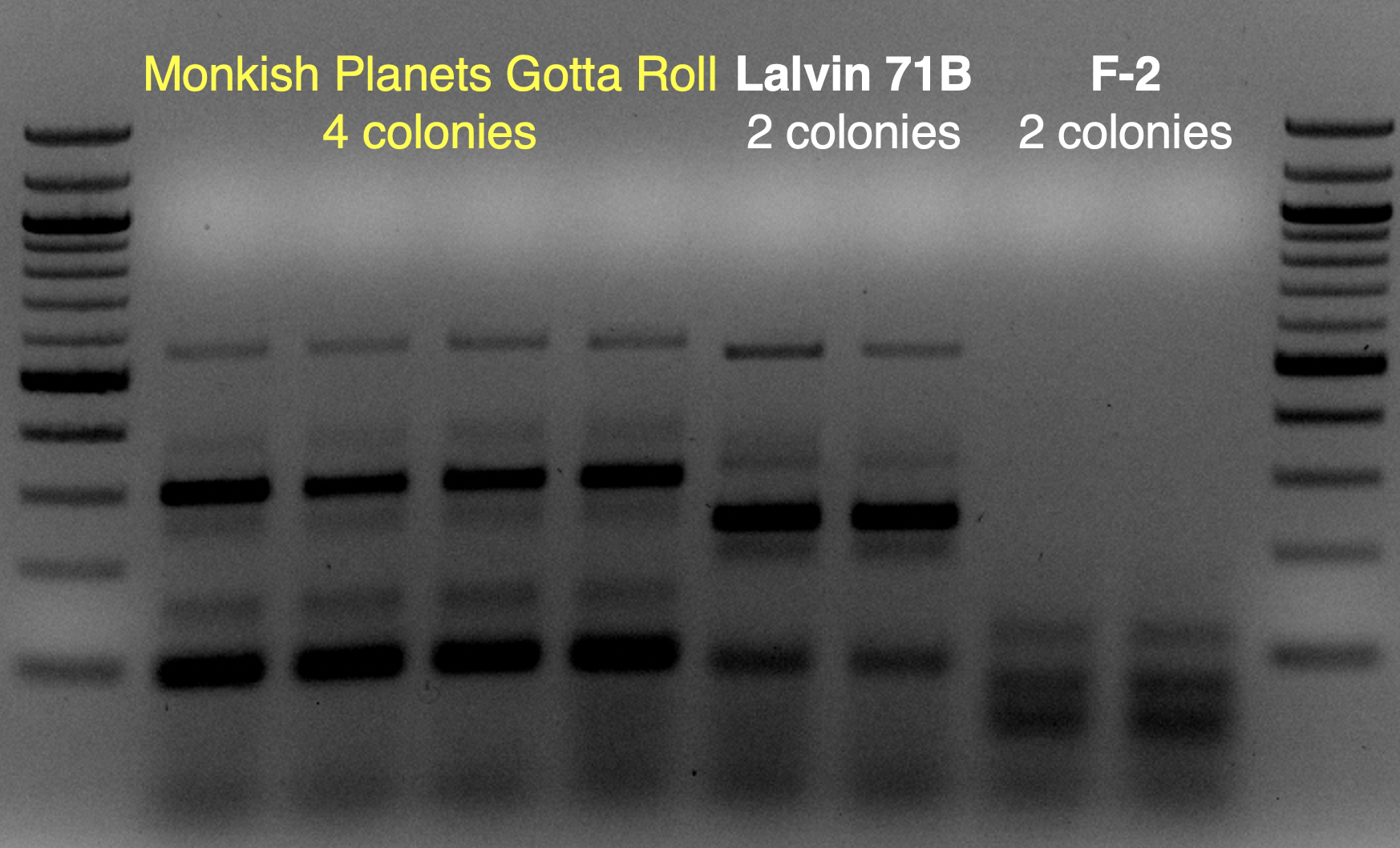
I can’t speak for @troxerX but I’ll be testing the slightly simplified version I proposed in my original post. Should be starting it in a few weeks. I don’t have the professional setup @echoALEia has but I’ll focused on minimizing oxidation during the blending process.Have you tested this yet, or are planning to?
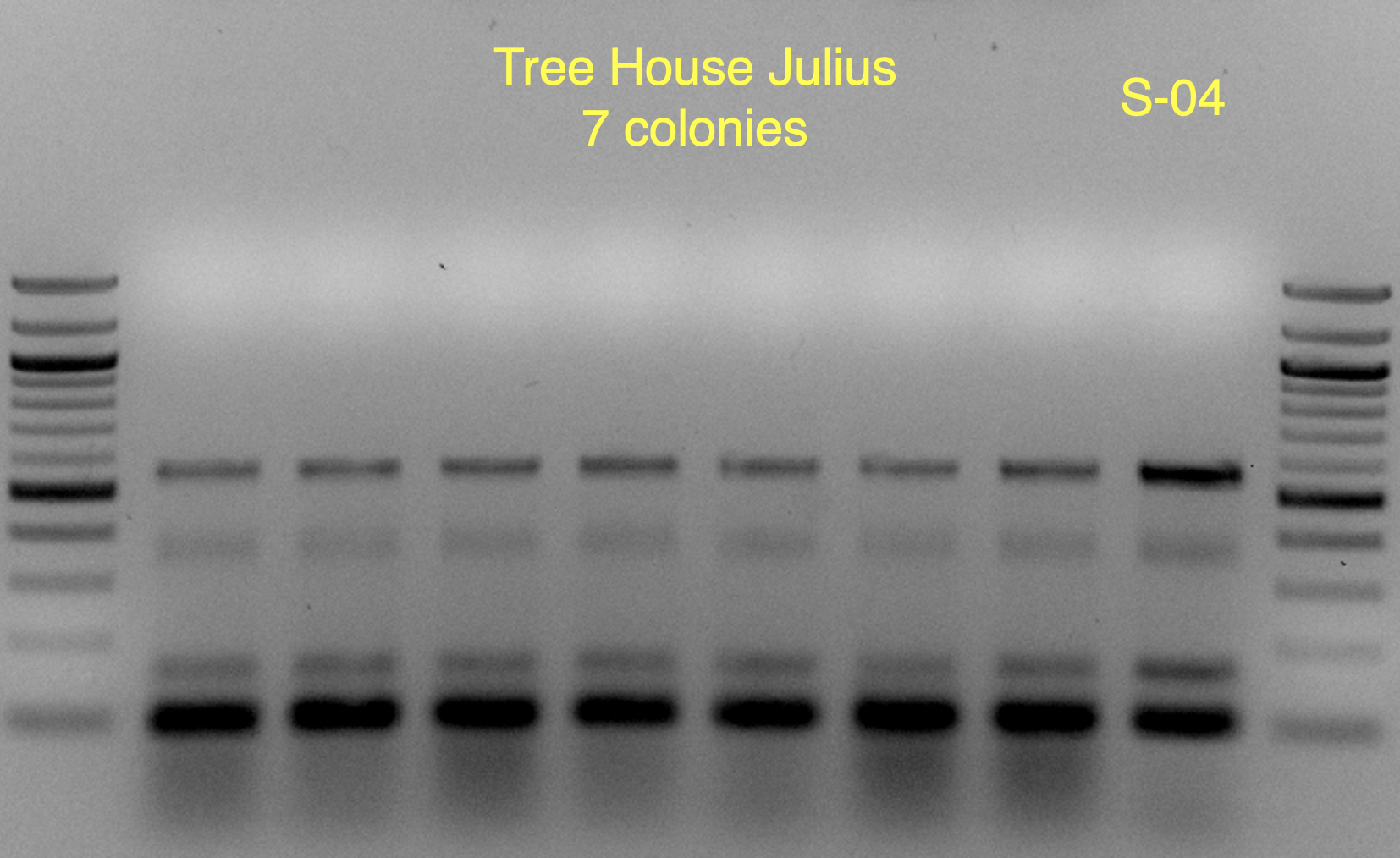
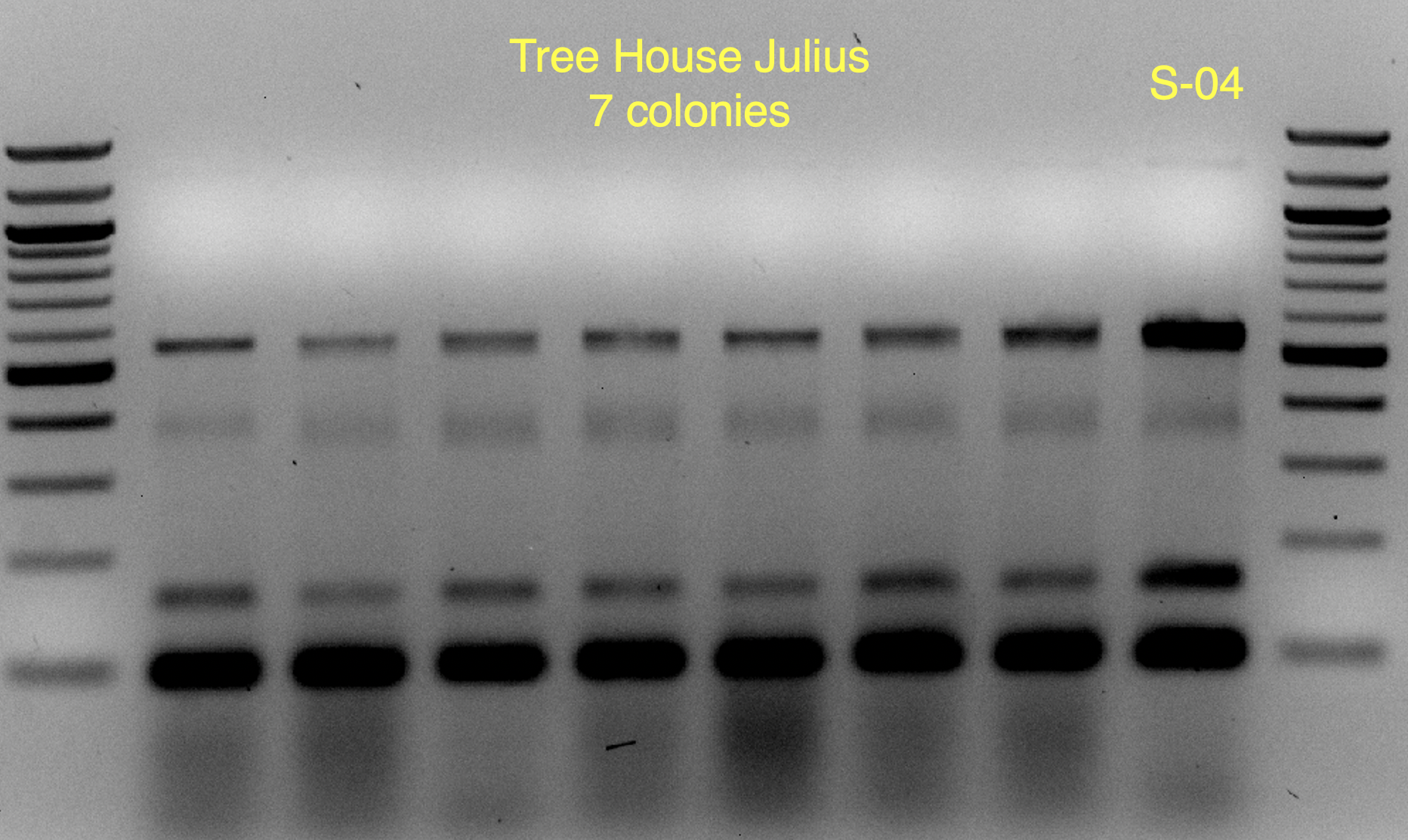
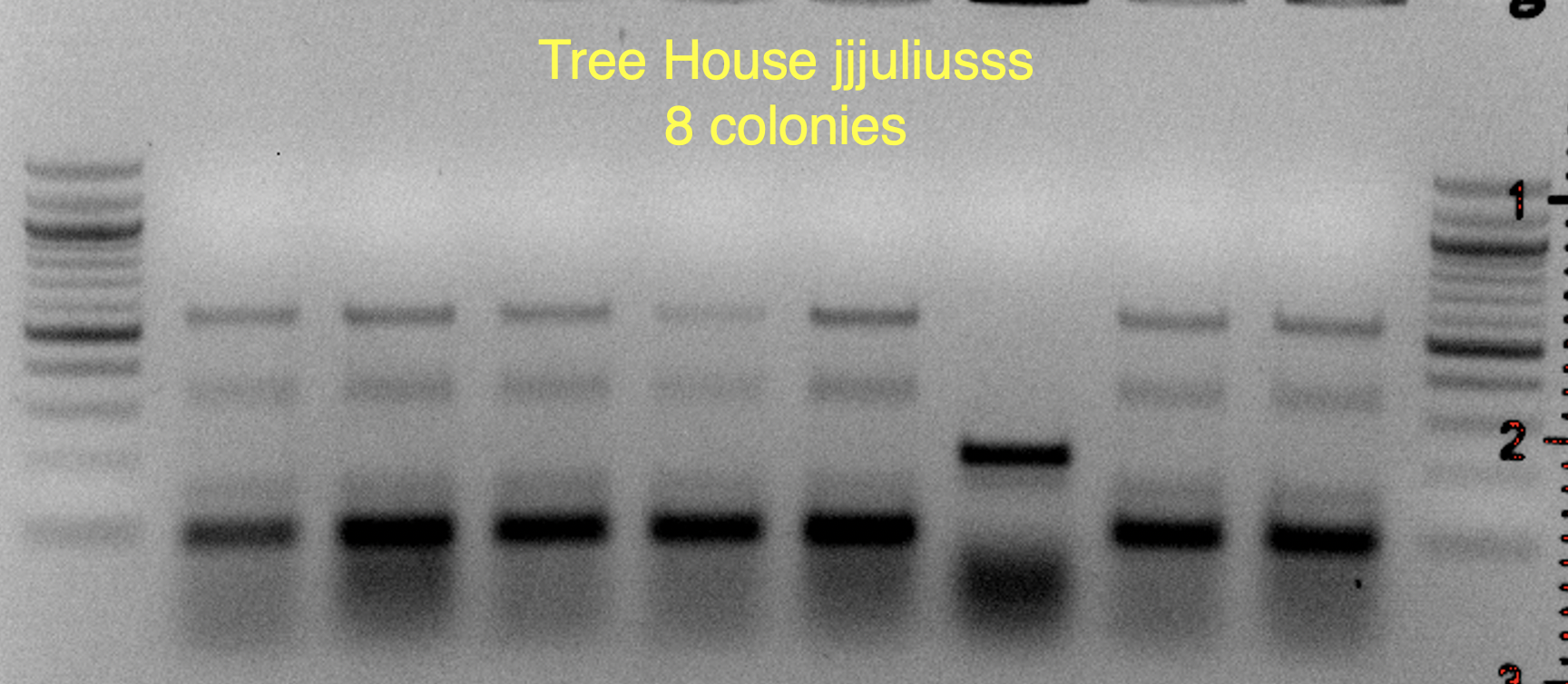
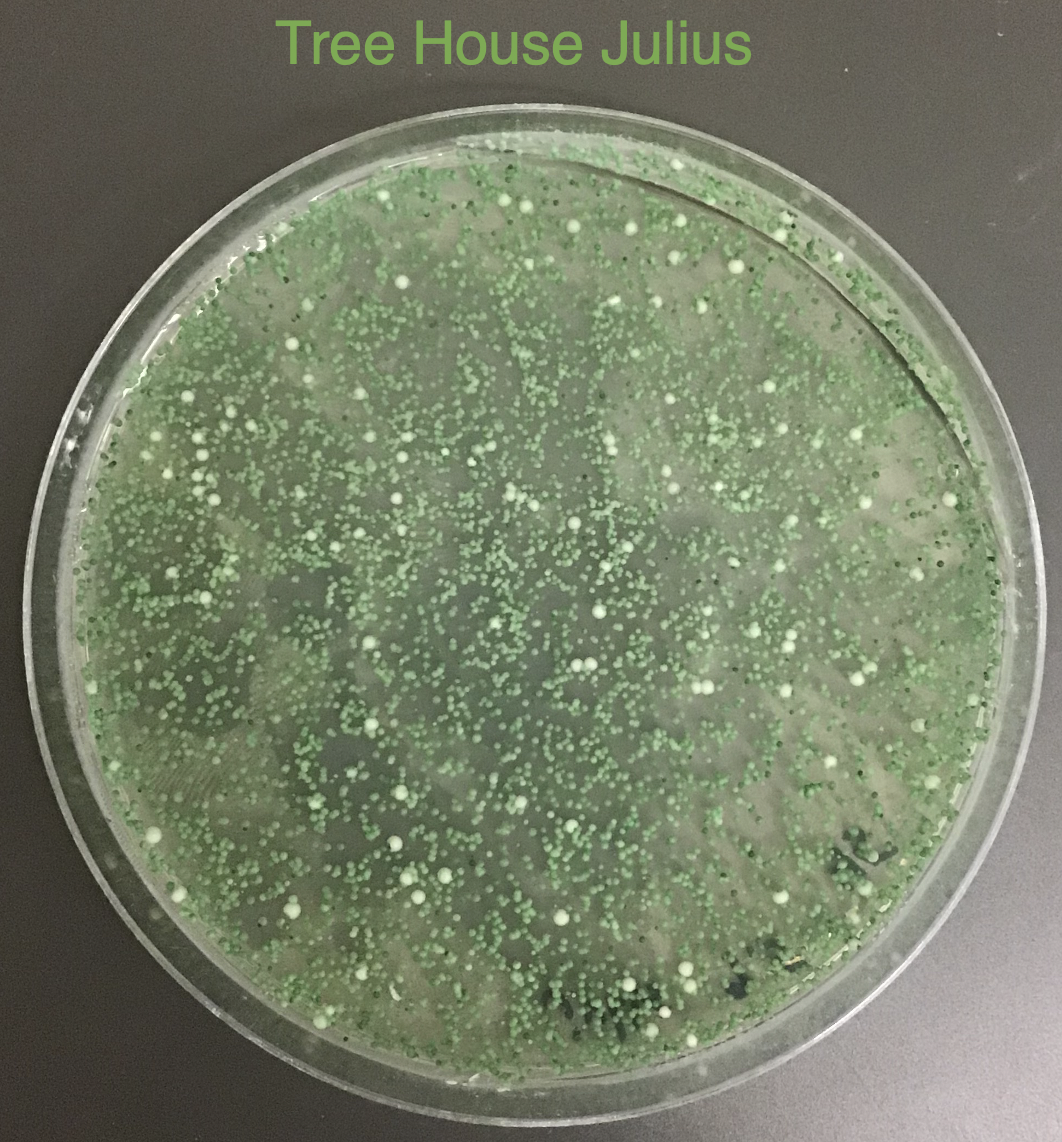
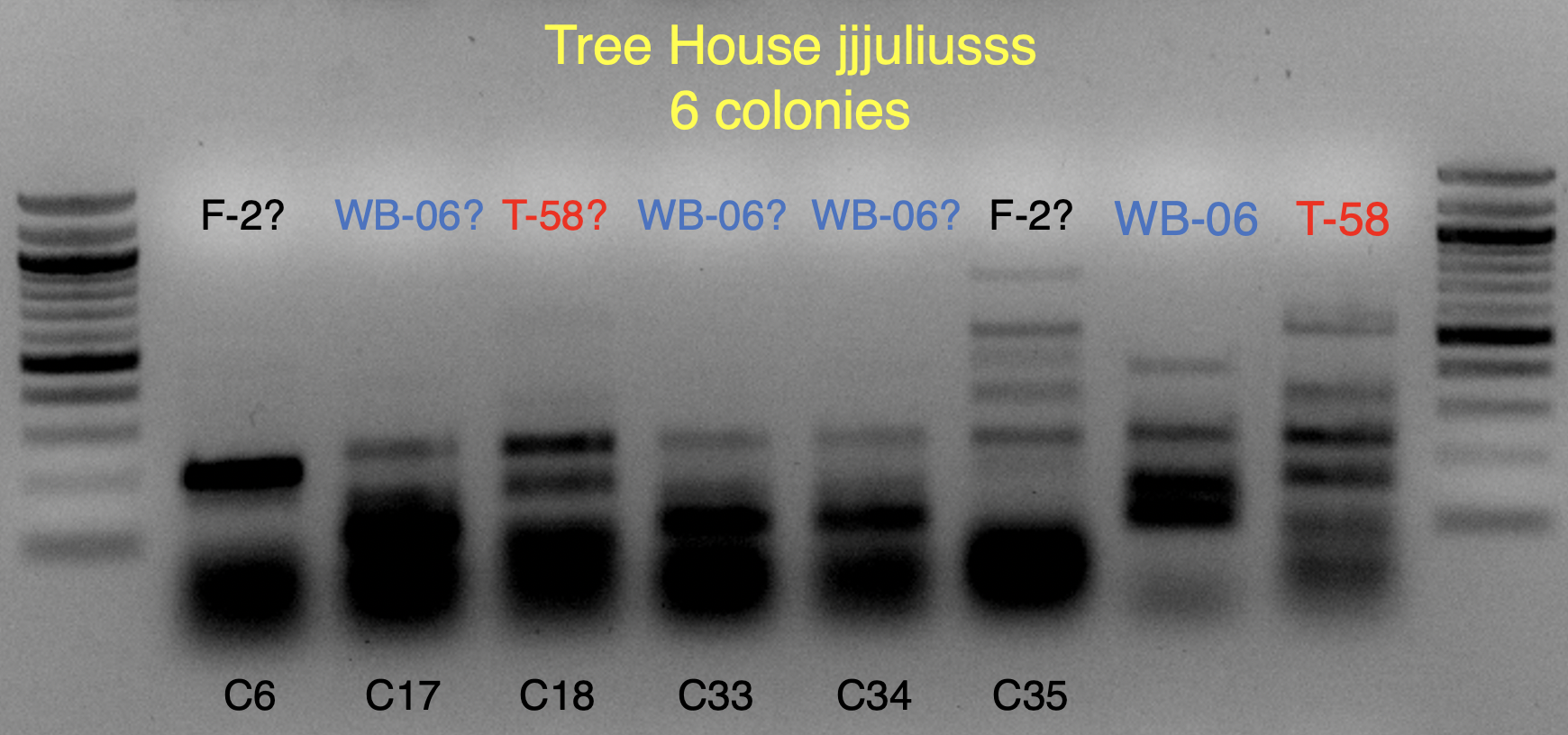
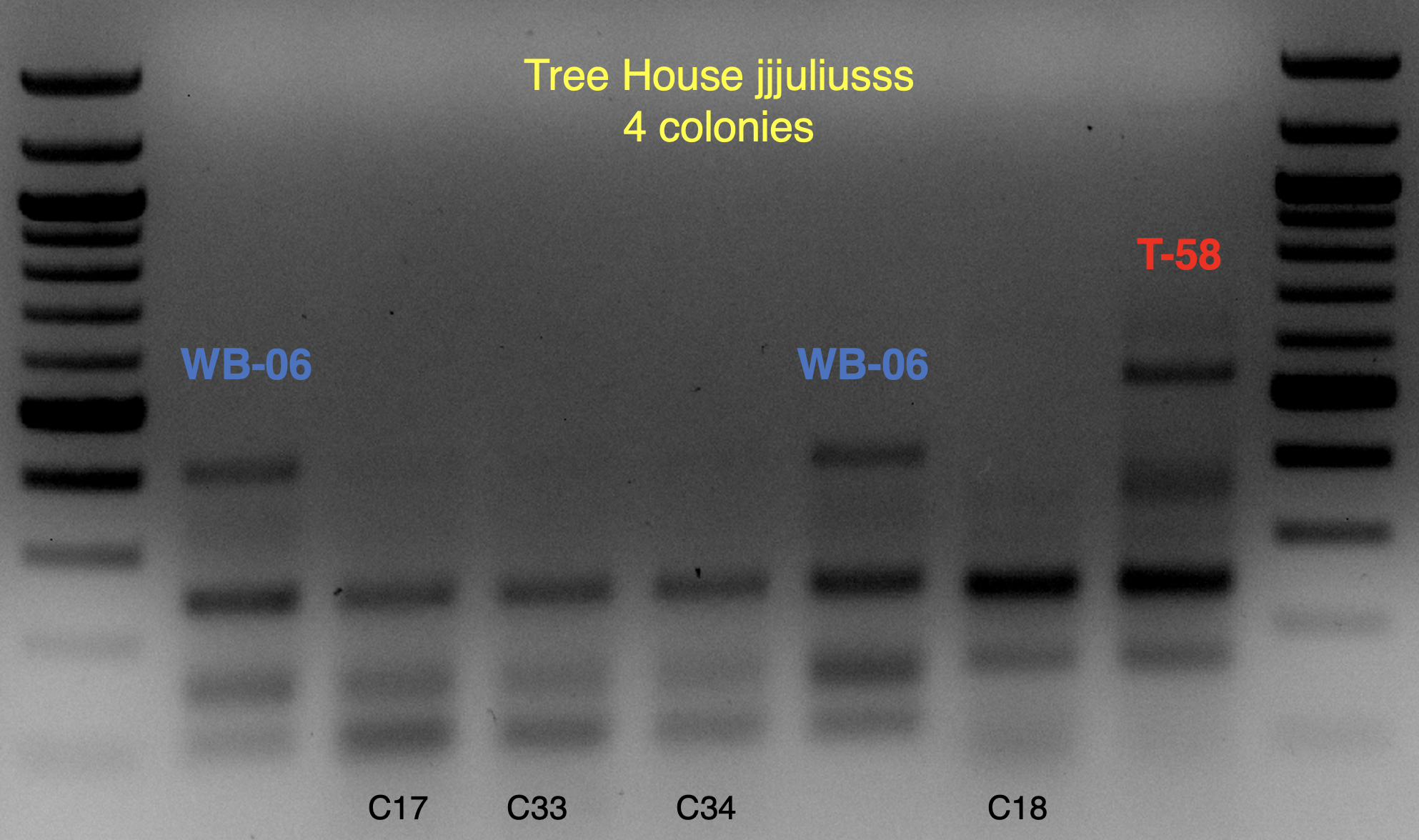
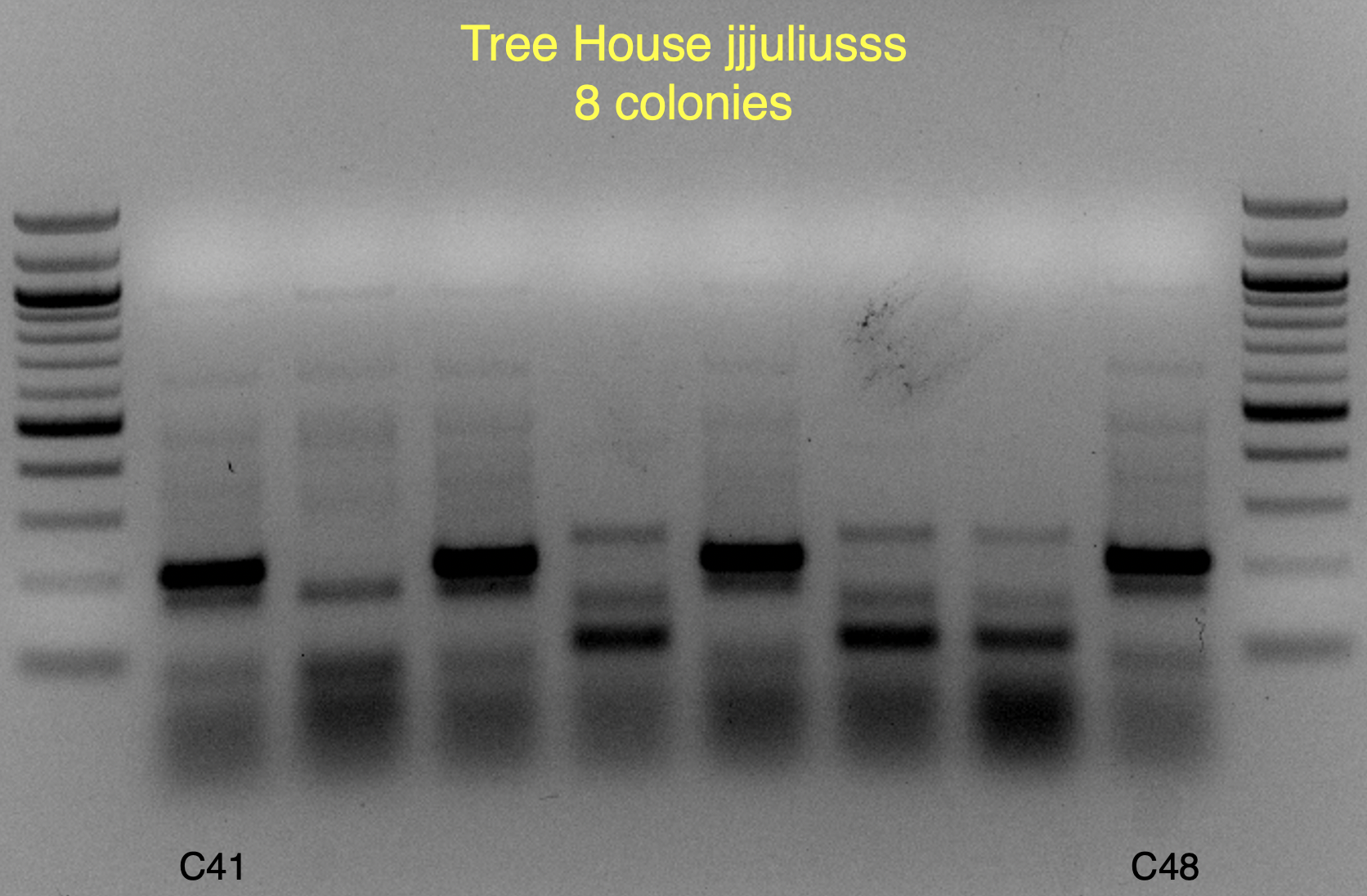
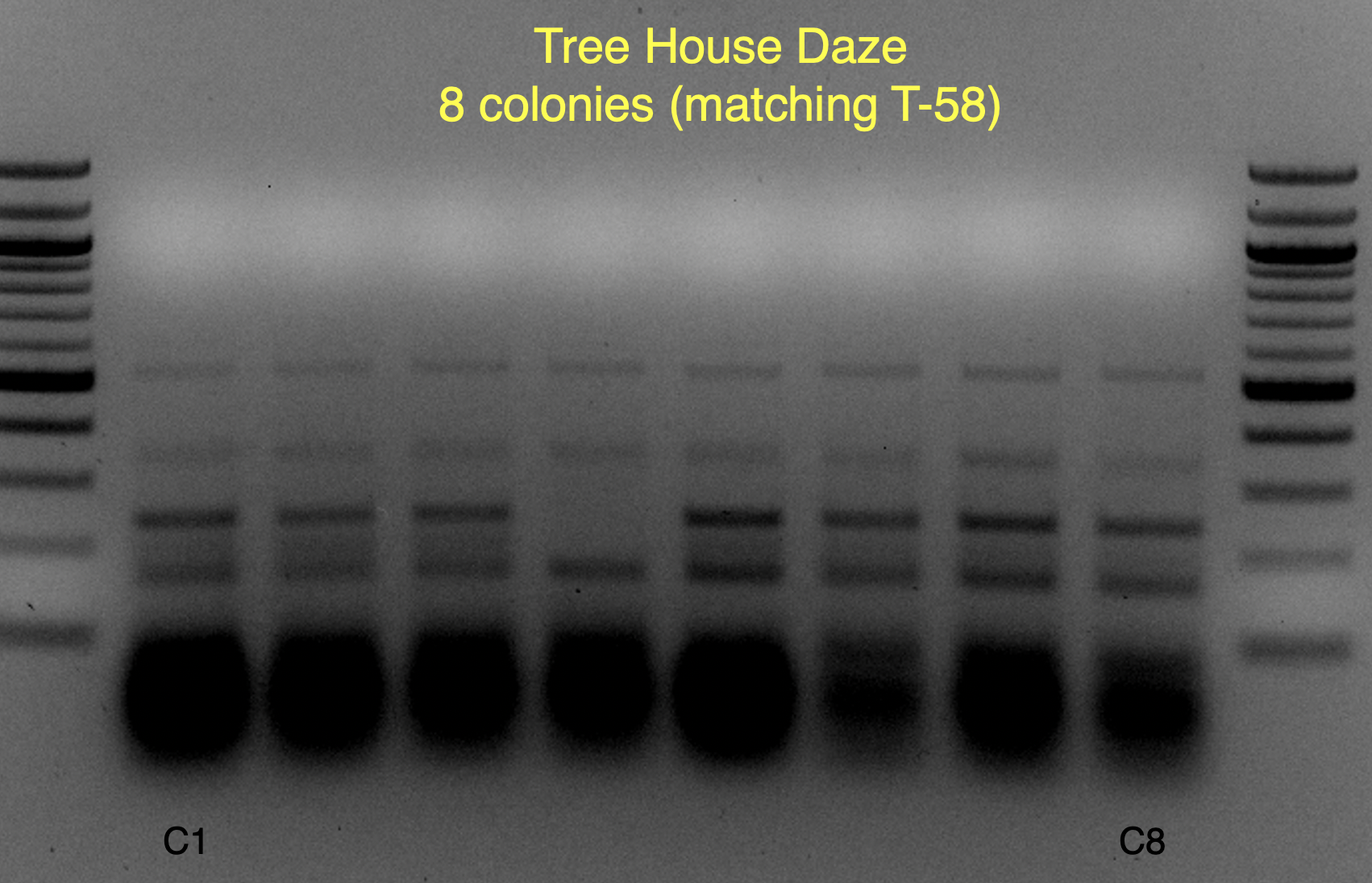
Based on the use of Brite Tanks at TH, and how we’ve seen the randomness of pitching a yeast blend all at once, I’m not firmly in the camp multiple worts are being fermented and used until we have evidence otherwise.
















![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)