My second to last batch was an IPA and tasted great, best beer yet. (My neighbor even liked it and he is a bud light drinker. In hind sight, I think my lack of water chemistry kept the IBU's muted for him)
Last batch was a double IPA and is very drinkable, but seems "ehhh". Not as bright as the commercial version.
Last week I went to Hyvee (grocery store) and bought a mix n match 6 pack. I noticed a lot of the commercial beers seemed pretty tart and crisp.
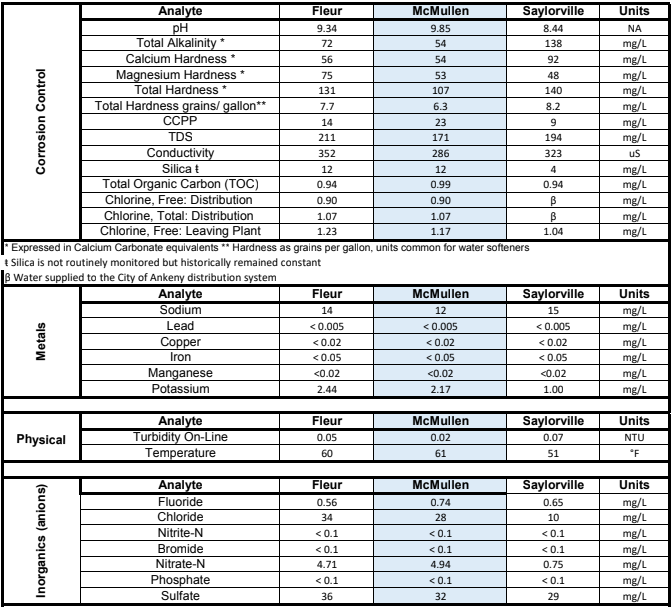
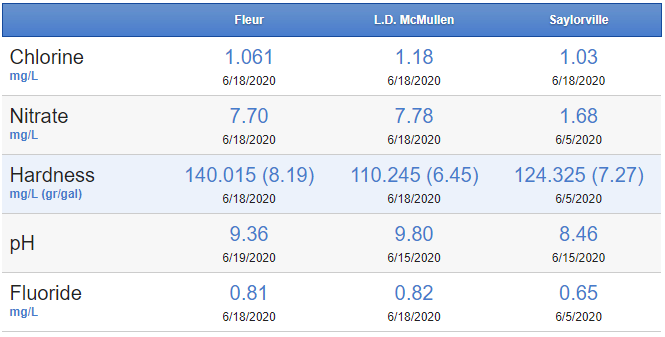
Anyways, started connecting the dots that my dad said the drinking water at my house is a lot harder than his. Hardness is probably correlated to being more basic and less acidic... I probably have a pH issue. So now I'm wanting to jump into water chemistry as my next improvement/step in brewing. I googled to see if my city (Des Moines, IA) posted a water analysis online and found this (below). So my question is, is this enough to start playing with water chemistry or do I need to quit being so cheap and order a water report to test my tap water (will do it this winter if I can wait). In the chart below, I believe I get water from the "Fleur" facility.
Last batch was a double IPA and is very drinkable, but seems "ehhh". Not as bright as the commercial version.
Last week I went to Hyvee (grocery store) and bought a mix n match 6 pack. I noticed a lot of the commercial beers seemed pretty tart and crisp.
Anyways, started connecting the dots that my dad said the drinking water at my house is a lot harder than his. Hardness is probably correlated to being more basic and less acidic... I probably have a pH issue. So now I'm wanting to jump into water chemistry as my next improvement/step in brewing. I googled to see if my city (Des Moines, IA) posted a water analysis online and found this (below). So my question is, is this enough to start playing with water chemistry or do I need to quit being so cheap and order a water report to test my tap water (will do it this winter if I can wait). In the chart below, I believe I get water from the "Fleur" facility.








![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)