In another thread Matt wondered about the purity of things like pickling lime and sodium bicarbonate. That thread has been pulled far enough off topic that I thought it might be better to start a new thread here as this may generate some discussion.
The general supposition is that as lime is made by
CaCO3 + H2O + heat ----> Ca(OH)2 + CO2
(the heat is added first and then the water) that the reaction is reversible
CO2 + Ca(OH)2 ----> Heat + H2O + CaCO3
This is the reaction by which cement cures and I mentioned in the other thread that it has only been in recent years that BLM shut off the cooling water to the Hoover dam structure as the cement had finally completely cured. The supposition is that over time the picking lime we buy from the supermarket will transform at least partially back into limestone which means rather than adding the quick acting lime we are adding the slow acting chalk whose problems we are trying to avoid by adding lime instead. The question is "Does my lime contain any calcium carbonate and if so how much?" It has been suggested here many times that a simple test would be to pour vinegar (or other acid) over a sample and see if it fizzes. If it does then there is obviously some chalk in it. If we try to dissolve half a gram in a liter of water and we can't then it's clear that there is some CaCO3 in there, but how much? The key to finding out is alkalinity.
Take a sample of the powder, put it in a dish or beaker and bake it in the oven for a couple of hours to drive off any absorbed water. Remove the powder from the oven and cool in a dessicator if you have one but otherwise in a small sealed jar. When it is cool weigh out about 1/4 gram quickly and put into a beaker or flask. You don't need an exact amount but you do want to know as exactly as possible what that amount was. Add 200 ml DI water and a stir bar. The solubility of Ca(OH2) is 173 mg/100 mL which would be 340 mg/200 mL (at 20 °C) so 250 mg should dissolve completely in 200 mL. If the water turns clear after some stirring then the powder was mostly Ca(OH)2. If it does not clear then there is some calcium carbonate in it. Add 0.1 N acid* to the 100 mL until pH 4.3 is reached. You will have to be careful doing this as the reactions take time to complete. Add a small amount of acid and monitor the pH. It may drop precipitously and then creep back up. After it does this add another small increment of acid, stir and wait. pH will inch up again. Eventually the solution will begin to clear and you can continue the titration to pH 4.3 but go really slowly on the acid once the solution is clear as a little bit causes a large pH swing as you approach end point. When end point is reached the total alkalinity (200 mL of DI water and powder) in mEq is simply the 0.1 times the number of mL of 0.1 N acid you used. The alkalinity of the liter of water itself is 2.5/50 per liter and so you can subtract 0.2*2.5/50 = 0.001 and can subtract that from the acid used if you want but it shouldn't be significant. Assuming 250 mg of pure calcium hydroxide with molecular weight 74 you would need 2*250/74 = 6.75676 mEq (67.57 mL of 0.1N acid). Assuming 250 mg of pure calcium carbonate with molecular weight 100 you would need 2*.992*250/100 = 4.960 mEq (49.6 mL 0.1 N acid). For lime that has partially degraded to CaCO3 you will need something in between.
Now lets see what to expect for each 100 mg of powder assuming the powder is 100*x percent (by weight) calcium hydroxide with molecular weight 74 and 100*( 1 - x) percent calcium carbonate with molecular weight 100. The alkalinity of 100 mg of the powder is then
alk = 200*x/74 + 0.992*200*(1-x)/100
Remember that this is alkalinity in mEq (not ppm as CaCO3). The 200 factors are there because each millimole of the calcium hydroxide and calcium carbonate contributes 2 mEq of proton absorbing capacity. The factor 0.992 is there because 0.8% of carbonate ion remains as bicarbonate at pH 4.3. You can probably neglect that factor but we'll continue to carry it. Doing some algebra but leaving the numbers in place so you can see where the molecular weights go
x = (alk/2 - 0.992)/((100/74 - 0.992)
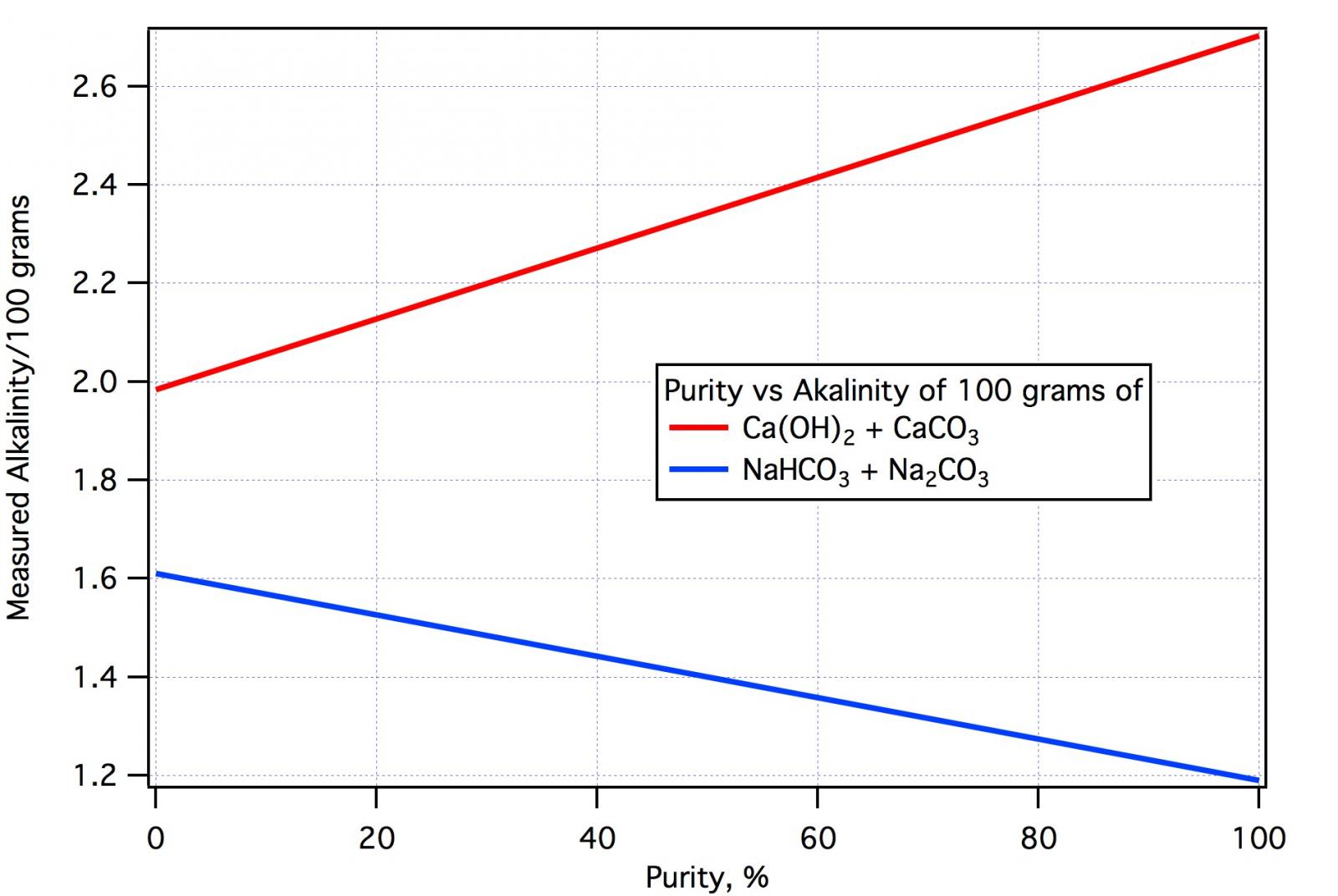
Remember that x is the fraction of calcium hydroxide in the powder and that alk is for 100 mg of powder (corrected for the water's alkalinity). If we had 100 mg of Ca(OH2) i.e. pure lime, alk would be 200/74 = 2.7027 mEq. If we had 100 mg of chalk (pure chalk) alk would be 0.992*200/100 = 1.984 mEq. Thus the difference between pure lime and pure chalk is approximately 0.7 mEq per 100 grams of powder. If titrating 100 mg of powder this would be a difference of only 7 mL of 0.1N acid. That's why we have done things 'per 100 mg of powder' thus giving you the option to use more powder and increase the sensitivity of the test. For 250 mg you would need 2.5 times more acid (i.e. there would be a difference of about 7*2.5 = 17.5 mL between pure and 0 percent lime) and small degradations become easier to see. Clearly the 17.5 mL difference covers 100% range so the sensitivity is about 5.7%/mL and, if the buret can be read to 0.1 mL then the sensitivity is about half a percent. To be clear: if a quantity of poweder other than 100 grams is used, divide the alkalinity by (amount_of_powder/100) and use that alkalinity in the formula for x.
Matt was also interested in sodium bicarbonate for which
alk = .992*100*x/84 + 0.992*200*(1-x)/124
per 100 mg powder. Here we are using the molecular weigh of the monohydrate form of sodium carbonate under the assumption that when it decomposes:
2 NaHCO3 ---> Na2CO3 + H2O + CO2
the water sticks around to hydrate the sodium carbonate and that the heating used to dry the sample before weighing is not sufficient to drive it off.
Again, sparing you the algebra we get a formula for the fraction of sodium bicarbonate based on the alkalinity per 100 mg.:
x = (alk/99.2 - 2/124)/(1/84 - 2/124)
Here for 100 % NaHCO3 we'd have an alkalinity of 1.19 and for 0% NaHCO3 alkalinity of 1.61 per 100 mg, a difference of 0.42 mEq equivalent to 4.2 mL of 0.1N acid. Using 250 mg would increase that to 10.5 for a basic sensitivity of about 10%/ml or 1% sensitivity for a buret readable to 0.1 mL.
Now, of course, if either the lime sample or bicarbonate sample is contaminated with, say, 10% calcium chloride or sodium chloride that is going to throw things way off. Evidence is that sodium bicarbonate, even as bought at the grocery store is quite pure (greater than 99%) so we wouldn't expect that to be the case with bicarb. OTOH with the lime I have no idea.
*0.1N acid is suggested because it is commonly sold for alkalinity measurement of water samples. Hach for example sells it by the liter for, I think, around $12.
The general supposition is that as lime is made by
CaCO3 + H2O + heat ----> Ca(OH)2 + CO2
(the heat is added first and then the water) that the reaction is reversible
CO2 + Ca(OH)2 ----> Heat + H2O + CaCO3
This is the reaction by which cement cures and I mentioned in the other thread that it has only been in recent years that BLM shut off the cooling water to the Hoover dam structure as the cement had finally completely cured. The supposition is that over time the picking lime we buy from the supermarket will transform at least partially back into limestone which means rather than adding the quick acting lime we are adding the slow acting chalk whose problems we are trying to avoid by adding lime instead. The question is "Does my lime contain any calcium carbonate and if so how much?" It has been suggested here many times that a simple test would be to pour vinegar (or other acid) over a sample and see if it fizzes. If it does then there is obviously some chalk in it. If we try to dissolve half a gram in a liter of water and we can't then it's clear that there is some CaCO3 in there, but how much? The key to finding out is alkalinity.
Take a sample of the powder, put it in a dish or beaker and bake it in the oven for a couple of hours to drive off any absorbed water. Remove the powder from the oven and cool in a dessicator if you have one but otherwise in a small sealed jar. When it is cool weigh out about 1/4 gram quickly and put into a beaker or flask. You don't need an exact amount but you do want to know as exactly as possible what that amount was. Add 200 ml DI water and a stir bar. The solubility of Ca(OH2) is 173 mg/100 mL which would be 340 mg/200 mL (at 20 °C) so 250 mg should dissolve completely in 200 mL. If the water turns clear after some stirring then the powder was mostly Ca(OH)2. If it does not clear then there is some calcium carbonate in it. Add 0.1 N acid* to the 100 mL until pH 4.3 is reached. You will have to be careful doing this as the reactions take time to complete. Add a small amount of acid and monitor the pH. It may drop precipitously and then creep back up. After it does this add another small increment of acid, stir and wait. pH will inch up again. Eventually the solution will begin to clear and you can continue the titration to pH 4.3 but go really slowly on the acid once the solution is clear as a little bit causes a large pH swing as you approach end point. When end point is reached the total alkalinity (200 mL of DI water and powder) in mEq is simply the 0.1 times the number of mL of 0.1 N acid you used. The alkalinity of the liter of water itself is 2.5/50 per liter and so you can subtract 0.2*2.5/50 = 0.001 and can subtract that from the acid used if you want but it shouldn't be significant. Assuming 250 mg of pure calcium hydroxide with molecular weight 74 you would need 2*250/74 = 6.75676 mEq (67.57 mL of 0.1N acid). Assuming 250 mg of pure calcium carbonate with molecular weight 100 you would need 2*.992*250/100 = 4.960 mEq (49.6 mL 0.1 N acid). For lime that has partially degraded to CaCO3 you will need something in between.
Now lets see what to expect for each 100 mg of powder assuming the powder is 100*x percent (by weight) calcium hydroxide with molecular weight 74 and 100*( 1 - x) percent calcium carbonate with molecular weight 100. The alkalinity of 100 mg of the powder is then
alk = 200*x/74 + 0.992*200*(1-x)/100
Remember that this is alkalinity in mEq (not ppm as CaCO3). The 200 factors are there because each millimole of the calcium hydroxide and calcium carbonate contributes 2 mEq of proton absorbing capacity. The factor 0.992 is there because 0.8% of carbonate ion remains as bicarbonate at pH 4.3. You can probably neglect that factor but we'll continue to carry it. Doing some algebra but leaving the numbers in place so you can see where the molecular weights go
x = (alk/2 - 0.992)/((100/74 - 0.992)
Remember that x is the fraction of calcium hydroxide in the powder and that alk is for 100 mg of powder (corrected for the water's alkalinity). If we had 100 mg of Ca(OH2) i.e. pure lime, alk would be 200/74 = 2.7027 mEq. If we had 100 mg of chalk (pure chalk) alk would be 0.992*200/100 = 1.984 mEq. Thus the difference between pure lime and pure chalk is approximately 0.7 mEq per 100 grams of powder. If titrating 100 mg of powder this would be a difference of only 7 mL of 0.1N acid. That's why we have done things 'per 100 mg of powder' thus giving you the option to use more powder and increase the sensitivity of the test. For 250 mg you would need 2.5 times more acid (i.e. there would be a difference of about 7*2.5 = 17.5 mL between pure and 0 percent lime) and small degradations become easier to see. Clearly the 17.5 mL difference covers 100% range so the sensitivity is about 5.7%/mL and, if the buret can be read to 0.1 mL then the sensitivity is about half a percent. To be clear: if a quantity of poweder other than 100 grams is used, divide the alkalinity by (amount_of_powder/100) and use that alkalinity in the formula for x.
Matt was also interested in sodium bicarbonate for which
alk = .992*100*x/84 + 0.992*200*(1-x)/124
per 100 mg powder. Here we are using the molecular weigh of the monohydrate form of sodium carbonate under the assumption that when it decomposes:
2 NaHCO3 ---> Na2CO3 + H2O + CO2
the water sticks around to hydrate the sodium carbonate and that the heating used to dry the sample before weighing is not sufficient to drive it off.
Again, sparing you the algebra we get a formula for the fraction of sodium bicarbonate based on the alkalinity per 100 mg.:
x = (alk/99.2 - 2/124)/(1/84 - 2/124)
Here for 100 % NaHCO3 we'd have an alkalinity of 1.19 and for 0% NaHCO3 alkalinity of 1.61 per 100 mg, a difference of 0.42 mEq equivalent to 4.2 mL of 0.1N acid. Using 250 mg would increase that to 10.5 for a basic sensitivity of about 10%/ml or 1% sensitivity for a buret readable to 0.1 mL.
Now, of course, if either the lime sample or bicarbonate sample is contaminated with, say, 10% calcium chloride or sodium chloride that is going to throw things way off. Evidence is that sodium bicarbonate, even as bought at the grocery store is quite pure (greater than 99%) so we wouldn't expect that to be the case with bicarb. OTOH with the lime I have no idea.
*0.1N acid is suggested because it is commonly sold for alkalinity measurement of water samples. Hach for example sells it by the liter for, I think, around $12.















































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)









