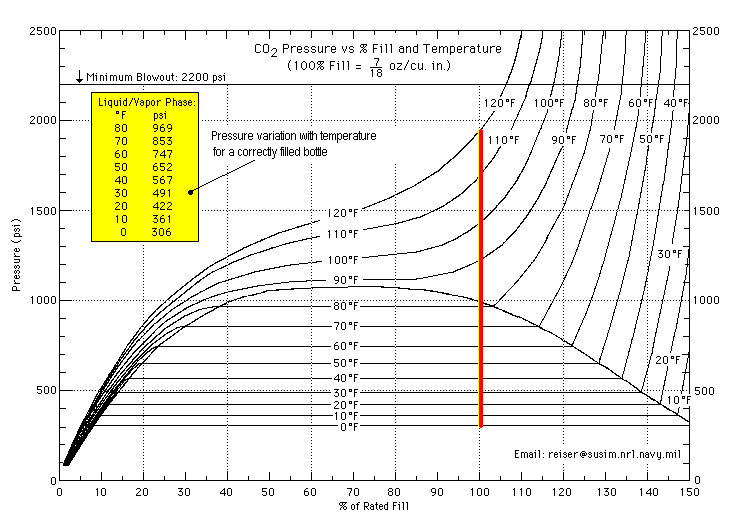
Oxygen (& nitrogen, hydrogen, helium, argon, etc.) all remain as gases when compressed, and the pressure is proportional to the amount of gas in the cylinder. CO2 is an odd duck, as it liquefies when compressed, so in a full cylinder you have mostly liquid CO2, as well as some gas in the headspace. Thus you have liquid and gas in equilibrium with each other. Whenever this is the case, the pressure depends only on the temperature of the CO2, not how much there is. So, changing the temperature of the CO2 cylinder will change the pressure. When the liquid is gone, then the CO2 gas behaves more other gases, and the pressure is proportional to the amount of CO2 in the cylinder. So, when the gauge starts dropping (at constant temperature) that means all the liquid CO2 is gone, and your tank is almost empty.
Brew on








![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)