macaronijones
Well-Known Member
- Joined
- Apr 1, 2016
- Messages
- 80
- Reaction score
- 11
I've been reading a lot about various ions, ratios, etc. with regards to water profiles and find myself just getting more confused, too many variables at play.
I've done about four all grain BIAB batches so far and while I have been generally been pleased with the results, each one seems to have a residual astringent aftertase. I've done a hefe, a black IPA, a Irish red, and an american wheat, so not just one style. It's a drying/puckering sensation at the back of the throat, I think "astringency" seems to be the best description, and I'm wondering if it has to do with my water.
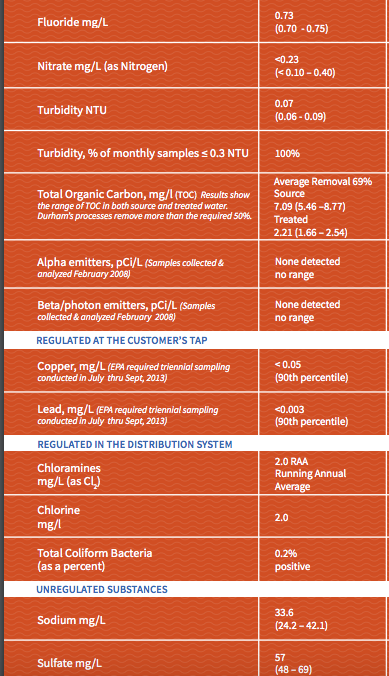
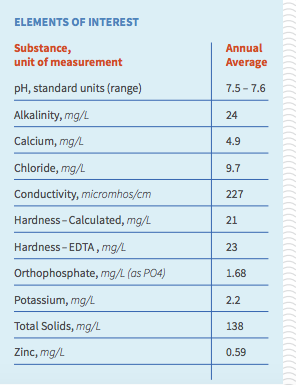
After doing some reading about alkalinity and astringency I thought that was most certainly the culprit, but after reviewing my local water report (alkalinity 24 mg/l) it seems that's unlikely. The rest of the relevant sections of my water report are attached.
So for those with more experience interpreting these, is there anything in there that could cause this sensation, or should I start considering other parts of my process (for completeness sake, I do full volume BIAB at recommended temps with no sparge, ferment at about 66)? Thanks in advance.
I've done about four all grain BIAB batches so far and while I have been generally been pleased with the results, each one seems to have a residual astringent aftertase. I've done a hefe, a black IPA, a Irish red, and an american wheat, so not just one style. It's a drying/puckering sensation at the back of the throat, I think "astringency" seems to be the best description, and I'm wondering if it has to do with my water.
After doing some reading about alkalinity and astringency I thought that was most certainly the culprit, but after reviewing my local water report (alkalinity 24 mg/l) it seems that's unlikely. The rest of the relevant sections of my water report are attached.
So for those with more experience interpreting these, is there anything in there that could cause this sensation, or should I start considering other parts of my process (for completeness sake, I do full volume BIAB at recommended temps with no sparge, ferment at about 66)? Thanks in advance.





















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)






































