- Joined
- Jun 2, 2008
- Messages
- 64,955
- Reaction score
- 16,524
Tap water report from Ward's:
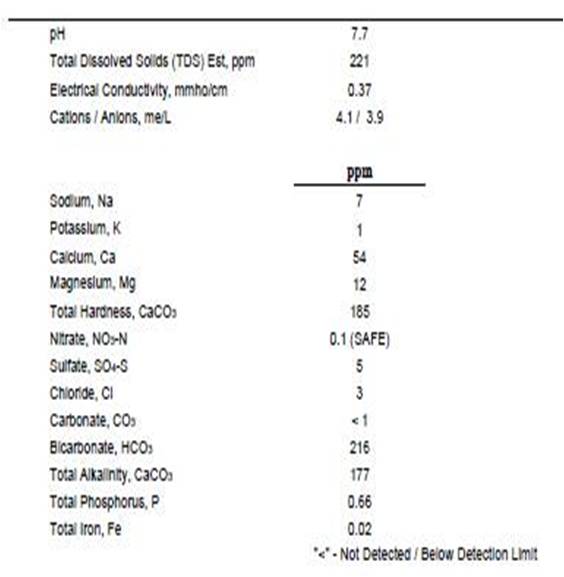
Grist
10 lbs 2-row
1 lb Munich
0.75 lb 60L
0.5 lb flaked barely
1.3 qt/lb mash ratio
Mash temp 153*F for 60 minutes
post-mash pH without "5.2" = 5.45 (taken by putting probe into mash)
post-mash pH with "5.2" = 5.18 (taken by putting probe into vessel collecting sparge water)
mid-sparge pH without "5.2" = 5.82 (taken by putting probe into mash)
mid-sparge pH with "5.2" = 5.29 (taken by putting probe into vessel collecting sparge water)
pH meter: http://www.marinedepot.com/ps_viewi...tm_campaign=adwordsfroogle&utm_content=HN1151
pH meter testing (room temp, 7.0 solution)

pH meter testing (room temp, 4.0 solution)

pH meter testing (~167*F, 4.0 solution)

pH meter testing (~164*F, 7.0 solution)

pH meter testing (~54*F, 4.0 solution)

pH meter testing (~50*F, 7.0 solution)


Grist
10 lbs 2-row
1 lb Munich
0.75 lb 60L
0.5 lb flaked barely
1.3 qt/lb mash ratio
Mash temp 153*F for 60 minutes
post-mash pH without "5.2" = 5.45 (taken by putting probe into mash)
post-mash pH with "5.2" = 5.18 (taken by putting probe into vessel collecting sparge water)
mid-sparge pH without "5.2" = 5.82 (taken by putting probe into mash)
mid-sparge pH with "5.2" = 5.29 (taken by putting probe into vessel collecting sparge water)
pH meter: http://www.marinedepot.com/ps_viewi...tm_campaign=adwordsfroogle&utm_content=HN1151
pH meter testing (room temp, 7.0 solution)

pH meter testing (room temp, 4.0 solution)

pH meter testing (~167*F, 4.0 solution)

pH meter testing (~164*F, 7.0 solution)

pH meter testing (~54*F, 4.0 solution)

pH meter testing (~50*F, 7.0 solution)



![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)






















































