triskelion
Well-Known Member
I'm looking for a basic explaination of reducing alkalinity by adding an acid to the water, lactic acid for example. From what I remember from school chemistry, a salt will form in this process. Is there a precipitate that needs to be removed or does it stay dissolved?
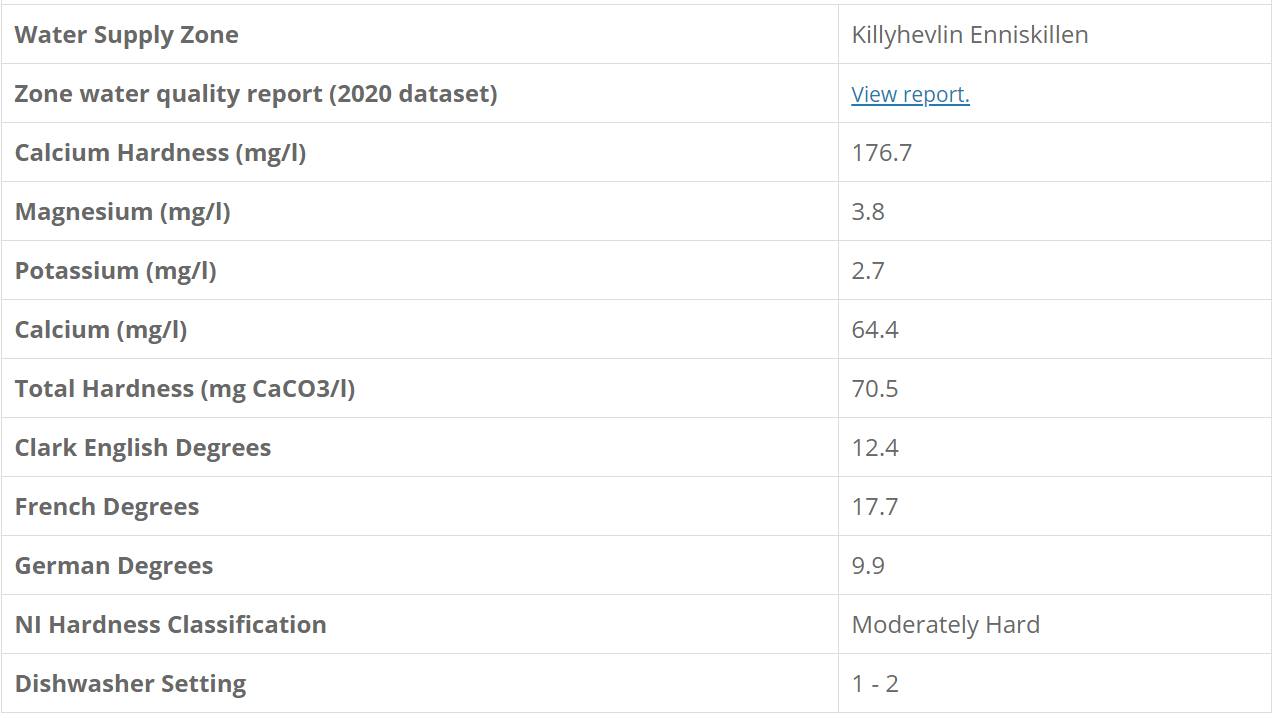
My water profile is the following: Ca 64, Mg 4, HCO 86, Na 22, Cl 22, S 110
I'd like to adjust to a very soft profile for lagers. Bru'n water suggests adding lactic acid will reduce calcium and bicarbonate significantly but I'm unfamiliar with doing this. Would it be the total water that is treated or is mash and sparge treated separately?
My water profile is the following: Ca 64, Mg 4, HCO 86, Na 22, Cl 22, S 110
I'd like to adjust to a very soft profile for lagers. Bru'n water suggests adding lactic acid will reduce calcium and bicarbonate significantly but I'm unfamiliar with doing this. Would it be the total water that is treated or is mash and sparge treated separately?
































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)