http://www.hach.com/pocket-pro-ph-tester-with-replaceable-sensor/product?id=17990686211
but don't necessarily get it from them as apparently they can be had for under $100.
Cool, thanks!

http://www.hach.com/pocket-pro-ph-tester-with-replaceable-sensor/product?id=17990686211
but don't necessarily get it from them as apparently they can be had for under $100.
I was quoted today on a hach at $93. I'm getting a discounted price from the supplier, so it's probably the same one you ordered. I'll wait for your feedback before buying, thanks
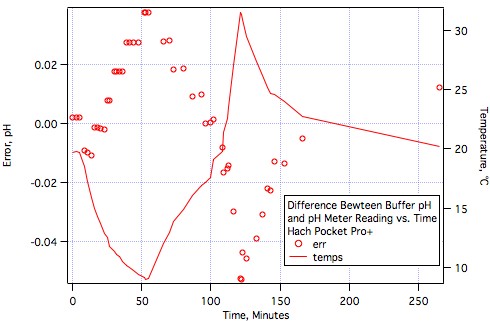
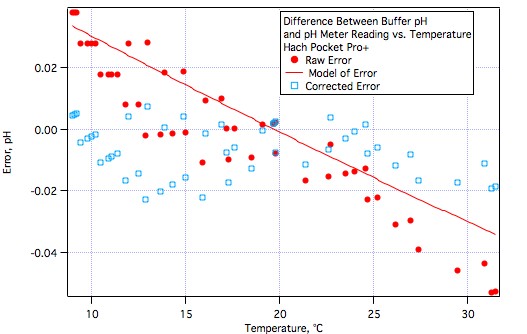
It's hard to stifle excitement but we really need to withhold final judgement until more brewers have tested them or at least worked with them.Very good news, AJ. Glad to see that meter performing well.
They aren't very forthcoming about design, that's for sure. In fact the manual isn't a font of information on much of anything. Nothing in it on long term storage, for example. I can't even figure out the intended market. Manual says 'general water testing'. What does that mean? Streams and rivers, waste and potable I guess. The box listed broader applications such as food industry, water water, brewing, beverages...I tried to ascertain the junction type for that meter, but couldn't find it mentioned on the Hach website or in their manual.
In following up with discussions on pH meters, I found that when dealing with 'dirty' water, it is best that the probe have a Double-Junction instead of Single-Junction. That was confirmed by my wastewater equipment supplier. So, I'm hoping that the Hach meter is a double-junction type. I was able to confirm that the Milwaukee MW 101 comes with a double-junction probe. Now I'm curious if those meters that seem to be more problematic are double- or single-junction probes?
On a related note, I see that you can pick up a Chinese probe with BNC connector for as little as $14. However, I'm pretty sure they are single-junction probes. I do see double-junction probes w/ BNC connector for under $40. I suppose that makes sense since the MW 101 meter and probe can be had for around $80. Don't scrimp here, get a double-junction probe with your meter.












MW100 and MW101 are supplied complete with a
MA911B/1 pH electrode, pH 7.01 20 mL sachet of calibration
solution, calibration screwdriver, 9V battery and
instructions.
MW102 is supplied complete with a MA911B/1 pH electrode,
MA830R stainless steel temperature probe, pH 4.01
and pH 7.01 20 mL sachet of calibration solution, 9V battery
and instructions.
Blarneybrew, would you mind sharing more about where/how you got this deal? I'm not finding the meter listed anywhere other than the hach site. Thanks!
I'll get back to you on it. I got a pricing through a water supplier's contact, and I never followed up on it as I was waiting for aj's feedback. This is all assuming it's the same tool as his also. It probably is.
I haven't. Even with my work discount at VWR, which can be substantial at times, there is no discount. Which makes me lean towards the Milwaukee one, which Amazon has for ~$75. So who to trust more, Martin or AJ's recommendation
AJ, what's the Pilsner malt used for this spreadsheet? (The one with DI pH of 5.62 at 20C)
It seems the wise thing to do is to calibrate and check stability over the first day of receiving the either product... I bet AJ has a procedure in mind as I write this!
Any manufacturer of anything will have lemons slip through his QT. It's a question of how many rinse cycles you have to go through. If it takes 5 shots to get a working electrode (and it did for me once many years back) you have a POS.Then return it if it isn't stable enough for use, and either get a duplicate replacement or the alternative vendor's equivalent, rinse and repeat.
The question is as to whether you are one of a few, a small majority or a large majority. As I mentioned earlier here, I think, it is not only a question of what the average review is but of how the individual reviews are distributed.Count me as a happy Milwaukee user, but probable I got lucky...
Actually maybe I mixed things up when I was reading one of AJ's posts. I had googled Crisp Maris Otter DI pH and found one of this posts where it mentioned the Maris Otter being 5.6 (not surprising given the extra roasting). He mentioned Weyermann in the same post as being 5.75.
That's to be expected of the English 'pale' malts as they are darker than the Pilsner malts. The 5.62 for Weyermann's is definitly unusual for them. And believe me, I have checked that result!
Wonder if you have checked the Bloods Maris Otter (sorry, couldn't resist).
I've done a few test mashes of English base malts. My last 5 or so beers used Simpson's Golden Promise and a test mash result of that in DI (room temp) was 5.56. Crips Maris Otter was very close to that also. I use 5.6 in EZ water for these malts and measure mash pH's reasonably close, usually a little lower than the prediction and account for that trend in planning because it's easy to correct with acid if required/desired.
Thanks for the info! It sounds like, pH-wise, it'll be a drop in replacement for Rahr 2-row...though I'll have to check.
This is definitely the right approach. Do the following in addition to the DI test. Add about 10 mEq of acid per kg of malt and measure the pH. Thus if you weigh out 50 grams of malt (1/20th of a kilo) you'd need 0.5 mEq of acid i.e. 1/2 mL of 1 N acid or 1 mL of 0.5 N acid (which may be easier to measure out with, for example, a syringe). You can make 1 N lactic acid by putting 8.48 ml of 88% lactic acid into a cylinder and making up to 100 mL with RO or DI water (DI preferred) or half normal by making up to 200 mL. Palmer's book has dilutions for other acids. If you do this test the amount of acid per kg divided by the pH shift is the average buffering capacity of the malt. For example if the DI mash pH is 5.7 and adding 1 mL of 0.5 N acid moves it to 5.2 and the malt sample weighed 50 grams then you have added 0.5*(1000/50) = 10 mEq/kg and the average buffering is -10/(5.7 - 5.5) = -50 mEq/kg-pH (with the minus sign being there because acids have a negative proton deficit). This ignores the non linearity (the buffering capacity seems to increase as you move away from the DI mash pH) but should be better than just guessing based on malt color.... I've tested and overwrite the EZ water # whenever using these and large portions of the grainbill:
This is definitely the right approach. Do the following in addition to the DI test. Add about 10 mEq of acid per kg of malt and measure the pH. Thus if you weigh out 50 grams of malt (1/20th of a kilo) you'd need 0.5 mEq of acid i.e. 1/2 mL of 1 N acid or 1 mL of 0.5 N acid (which may be easier to measure out with, for example, a syringe). You can make 1 N lactic acid by putting 8.48 ml of 88% lactic acid into a cylinder and making up to 100 mL with RO or DI water (DI preferred) or half normal by making up to 200 mL. Palmer's book has dilutions for other acids. If you do this test the amount of acid per kg divided by the pH shift is the average buffering capacity of the malt. For example if the DI mash pH is 5.7 and adding 1 mL of 0.5 N acid moves it to 5.2 and the malt sample weighed 50 grams then you have added 0.5*(1000/50) = 10 mEq/kg and the average buffering is -10/(5.7 - 5.5) = -50 mEq/kg-pH (with the minus sign being there because acids have a negative proton deficit). This ignores the non linearity (the buffering capacity seems to increase as you move away from the DI mash pH) but should be better than just guessing based on malt color.
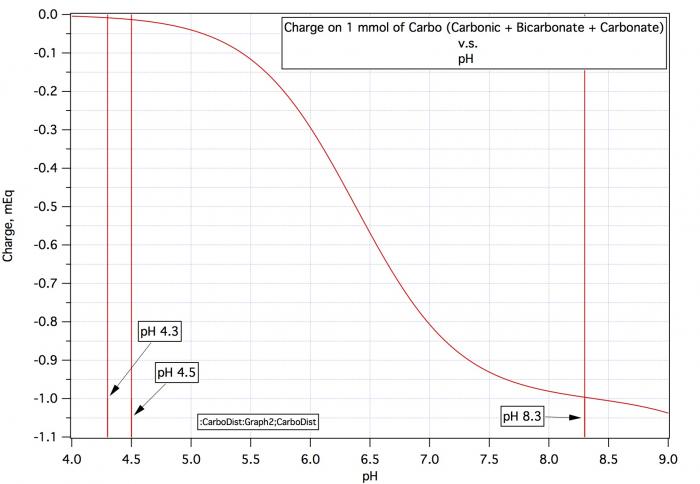
Now how to deal with the colored malts. The same way but you need a base of calibrated strength. That's hard to come by as calcium containing bases react with malt phosphate to release acid and sodium hydroxide solid picks up water and CO2 from the air very quickly. For acidic malts sodium carbonate should do. Make a 0.5 M solution of sodium bicarbonate by adding 8.4 grams to a mixing cylinder and making up to 100 mL. Grind up the dark malt, put 50 grams in a beaker, add DI water mix, hold at about 50°C for 25 minutes, remove some liquid, cool and measure pH. Now do the same again but add 1 mL of the 0.5 N sodium bicarbonate solution. Remove and cool a sample and check pH. Lets say that the DI pH was 4.9 and it rose to 5.4 (half a pH unit as before). As before the buffering capacity is the amount of acid neutralized divided by the pH change. To determine the amount of acid absorbed you need to know the normality of the 0.5 M bicarbonate solution. That comes from the curve below (which is also in the Water book on p 96). At final pH (5.4) the charge on 1 mmol of carbo is -0.1. The charge on 1 mmol of bicarbonate is -1.0. There has been a change of 0.9 and as the solution is 0.5 M the normality is 0.5*0.9 = 0.45 N. Thus the average buffering capacity of the colored malt is
0.45*(1000/50)/(5.4 - 5.6) = -45 mEq/kg-pH.
Now with these two buffering capacities you can easily calculate how much acid is required to lower the pH of base malt and how much acid is provided by the colored malt. Mash pH is the pH at which the two balance.
Just musing here....