arturo7
Well-Known Member
Fact or Opinion?
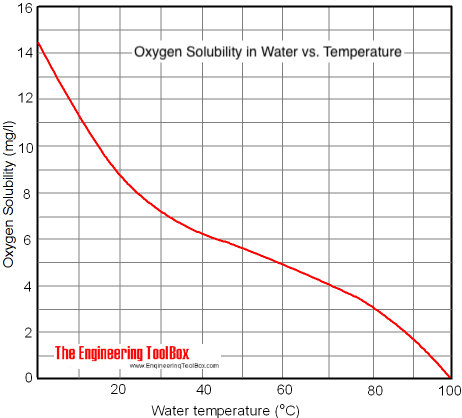
>Dissolved oxygen content of 8-16 PPM is ideal for yeast.
>8 PPM can be acheived with shaking, splashing or forced aeration (aquarium pump with stone).
>8 PPM cannot be exceeded without the use of pure oxygen.
Also, has anyone used one these DO measuring kits?
http://www.coleparmer.com/catalog/product_view.asp?sku=5300300
x
>Dissolved oxygen content of 8-16 PPM is ideal for yeast.
>8 PPM can be acheived with shaking, splashing or forced aeration (aquarium pump with stone).
>8 PPM cannot be exceeded without the use of pure oxygen.
Also, has anyone used one these DO measuring kits?
http://www.coleparmer.com/catalog/product_view.asp?sku=5300300
x























































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)

