- Joined
- Nov 26, 2013
- Messages
- 9,932
- Reaction score
- 24,122
How much time is saved during cool down by using frozen liquid (water, grapefruit juice, ...) vs just chilled liquid (say at 40*F)? Are there equations to estimate this?
Yes. Thermal gain from phase change, and thermal gain from two temp-diff vols of liquid. You gain more from phase change (frozen to liquid). You just need mass and temp of frozen amount, mass and temp of wort amount, and Google.
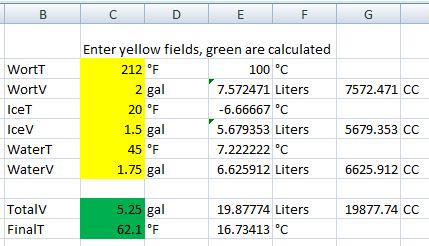
Example here
I also made something in Excel when I used to extract brew. I ended up never using ice as boiling, freezing, sanitizing containers, etc was just blech to me. I always just used tap or refrigerated tap water, cooling small 2g of boiling wort in sink bath first since the delta-temp of sink and boiling wort take delta-Q heat transfer very quick
Last edited:









![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)
















































