dawn_kiebawls
Lawncare and Landscaping enthusiast
- Joined
- Jun 10, 2017
- Messages
- 838
- Reaction score
- 519
I finally decided to sit down and figure out how to use BrunWater to build a profile from 100% distilled to brew a Flanders Red this weekend. When I pull up the pre determined water profiles they have a 'West Flanders' and a 'West Flanders Boiled' option. They also indicate that the 'boiled' profile is the profile once it has been 'boiled and decanted'.
So, my dumb questions is: Am I to assume that when they say 'boiled and decanted' that is the profile of the boiled/chilled wort going into the fermentor?? Or am I just completely out in the deep end here?
As I said, I've never dealt with water but am getting started to make sure this 1+ year aged beer will be worth the wait. I'm 99% sure that is what they're talking about but it seems weird they would add that to the database. On the screenshot I attached, I'm talking about lines 80/43 and 81/44
Thanks for any and all help. Cheers!
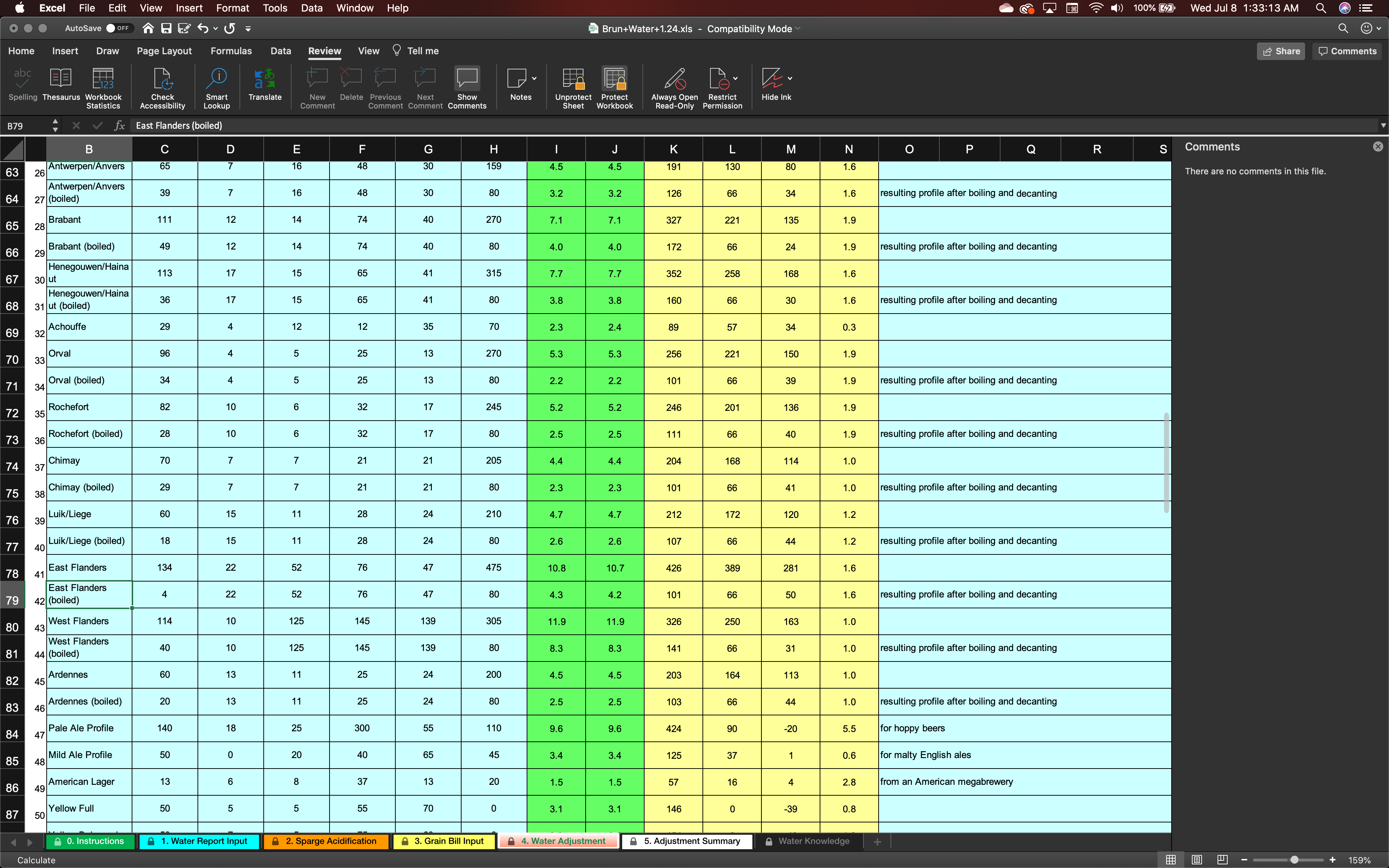
So, my dumb questions is: Am I to assume that when they say 'boiled and decanted' that is the profile of the boiled/chilled wort going into the fermentor?? Or am I just completely out in the deep end here?
As I said, I've never dealt with water but am getting started to make sure this 1+ year aged beer will be worth the wait. I'm 99% sure that is what they're talking about but it seems weird they would add that to the database. On the screenshot I attached, I'm talking about lines 80/43 and 81/44
Thanks for any and all help. Cheers!

Last edited:









![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)















































