New user to Bru'N Water, but Lord knows I love playing with numbers. Tweak the spreadsheet long enough and it starts to feel like reality!
Anyway, brewing a stout tomorrow with Denny's 1450 (rumored to floc slowly) so feel the need to get near the calcium target for Black Balanced profile. My tapwater chloride level is close to target, so both Gypsum and CaCl push either sulfite or chloride over target. I've chosen to do both to manage the ratio between the two. Is this wise, or would I be better off diluting and then building to keep chloride on target? (prefer just using tap water)
Here's the profile (if I know how to attach...):
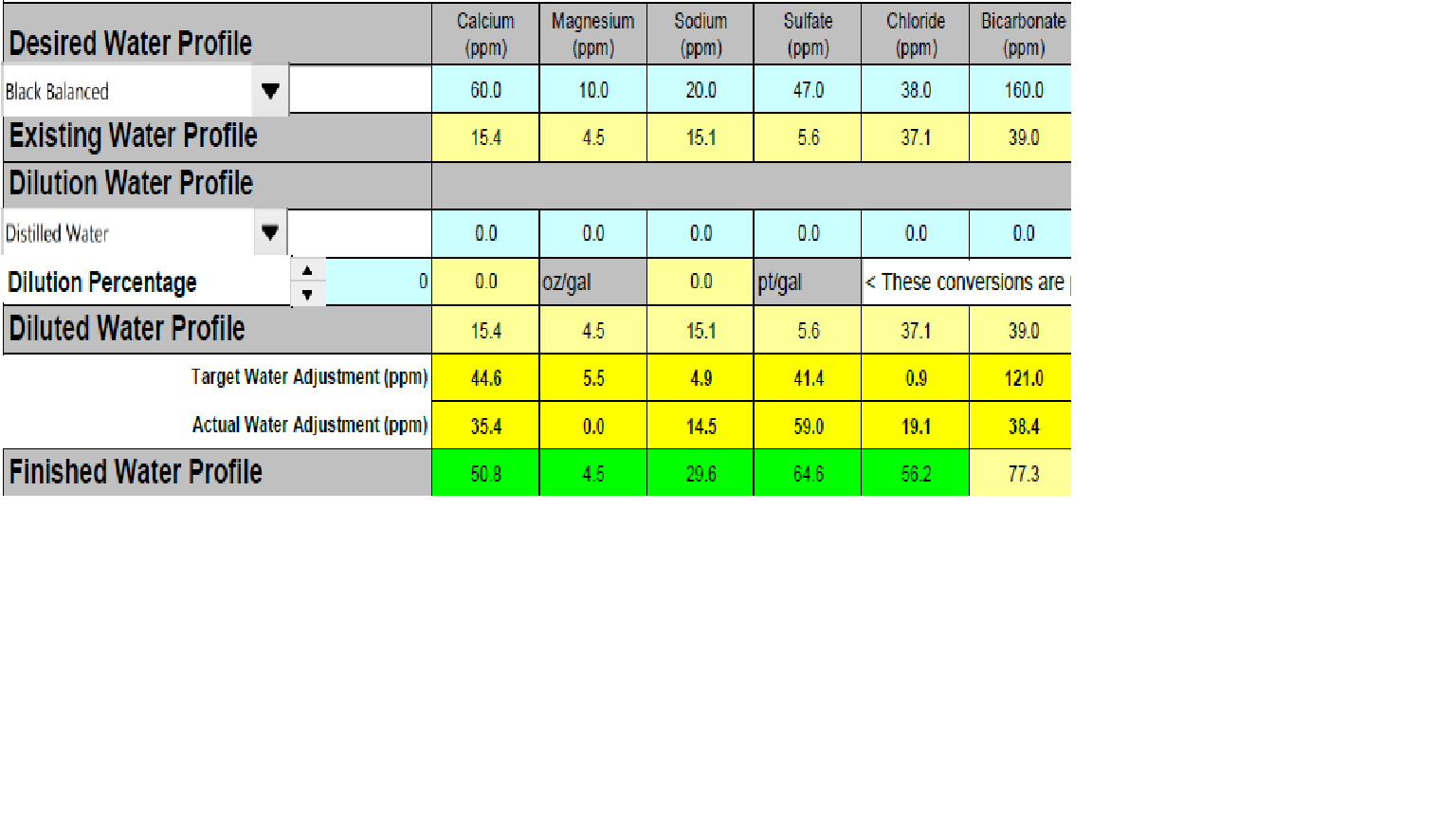
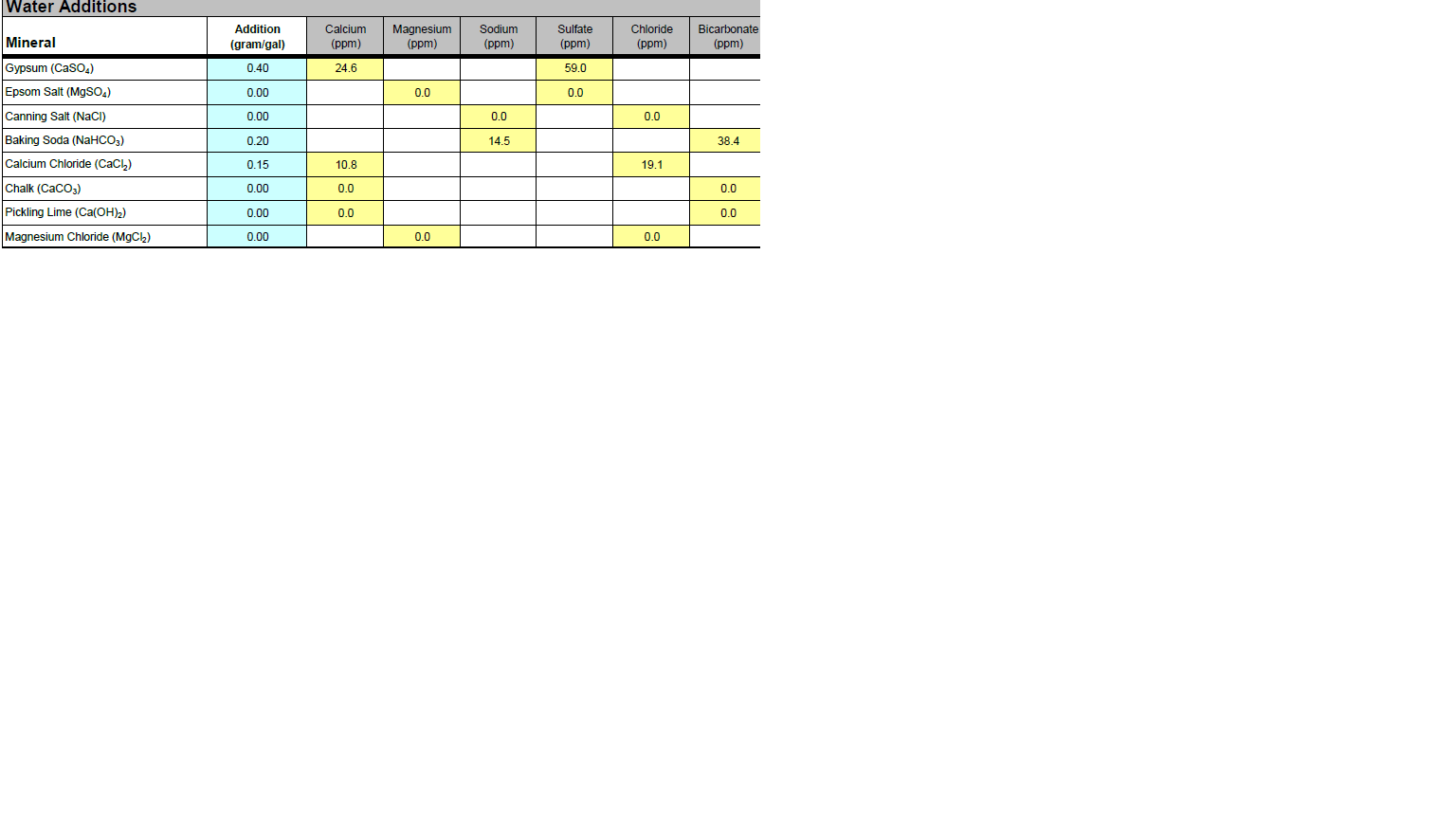
Anyway, brewing a stout tomorrow with Denny's 1450 (rumored to floc slowly) so feel the need to get near the calcium target for Black Balanced profile. My tapwater chloride level is close to target, so both Gypsum and CaCl push either sulfite or chloride over target. I've chosen to do both to manage the ratio between the two. Is this wise, or would I be better off diluting and then building to keep chloride on target? (prefer just using tap water)
Here's the profile (if I know how to attach...):










































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)



















