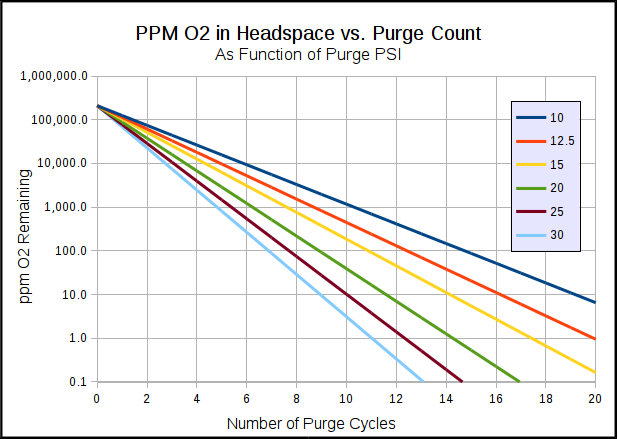
I don't know where I got the more head space thing from those graphs. And you're the only one talking about moles here. All of my comments and the links I posted referenced percentages. You say that converting from percent to moles is just simple number crunching, but you would definitely have to know the head space volume to do the conversion (which is why I used percent, as it's more universally applicable).
My "numbers" were just SWAGs as I wasn't trying to dig into any textbooks for gas-gas diffusion constants when this isn't in the Brew Science forum and I wasn't trying to over complicate this more than it needed to be. But fine, just to prove my point of how much the air mixes into a keg I dug one up, and this is the equation to calculate the penetration distance of diffusing gases:
D = √(Ct)
where
D is the distance diffused (cm),
C is the diffusion constant, and
t is time (s). The function assumes 1 atmosphere of pressure at room temp in a controlled environment.
For O2 diffusing into CO2,
C = 0.16 cm²/s
Plugging it in:
[
t=1] → [
D=0.4 cm]
[
t=60] → [
D=3.08 cm]
[
t=120] → [
D=4.38 cm]
Now extrapolate that into a real world situation. The A/C is on, the dog and kids are running around,
you're breathing, the cat gets nosy, whatever....you get the picture. Just removing (and replacing) the lid to the keg itself will push enough air into the keg to warrant a few extra purges. And likely you removed the lid because you want to put something in the keg, which will also pull O2 with it.
Is it tons of oxygen? No, I think we all agree on that. But to say it's not worthy of attention isn't correct. If the OP didn't care about oxidation, he wouldn't be here asking about how to reduce it in this instance. Perhaps you needn't purge any more than you would when kegging the beer initially, but I would say certainly not less either. I still hold that one or two purges will not void the oxygen you let in to less than the acceptable industry threshold.
And as I stated earlier...if the keg is going to be emptied within a week or two, it's highly unlikely the effects of oxidation, if any, would present themselves by the time it's gone.
Otherwise....we go through all this trouble to keep the dreaded O2 out of our beer throughout fermentation, transfers, etc, only to neglect it now, after all of that?

















































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)