Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
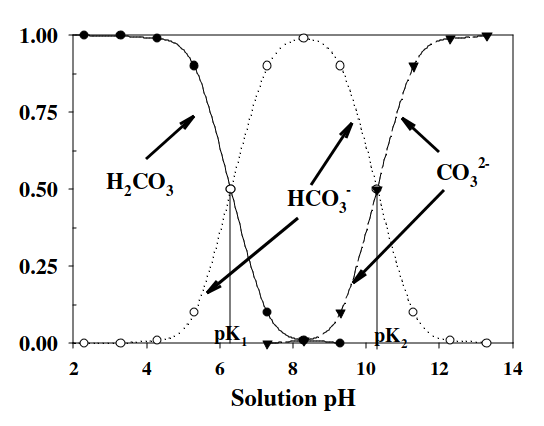
For natural water of pH 4.30:
Water's pH = 6.40 + log(mol fraction ratio of HCO3- to H2CO3)
4.30 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
-2.10 = log(mol fraction of HCO3- to H2CO3)
10^-2.10 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- = 0.00794 x 100 = 0.794%
mol fraction H2CO3 = (1-0.0.00794) x 100 = 99.206%
For natural water of pH 5.40:
Water's pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
5.40 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
-1.00 = log(mol fraction of HCO3- to H2CO3)
10^-1.00 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- = 0.100 = 10.0%
mol fraction of H2CO3 = 1-0.100 = 90.0%
For natural water of pH 6.40:
Water's pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
6.40 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
0.00 = log(mol fraction of HCO3- to H2CO3)
10^0.00 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- to H2CO3 = 1 = 50% HCO3- and 50% H2CO3
For natural water of pH 8.40:
Water's pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
8.40 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
2.00 = log(mol fraction of HCO3- to H2CO3)
10^2.00 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- to H2CO3 = 100 = 100% HCO3- and 0% H2CO3
Water's pH = 6.40 + log(mol fraction ratio of HCO3- to H2CO3)
4.30 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
-2.10 = log(mol fraction of HCO3- to H2CO3)
10^-2.10 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- = 0.00794 x 100 = 0.794%
mol fraction H2CO3 = (1-0.0.00794) x 100 = 99.206%
For natural water of pH 5.40:
Water's pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
5.40 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
-1.00 = log(mol fraction of HCO3- to H2CO3)
10^-1.00 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- = 0.100 = 10.0%
mol fraction of H2CO3 = 1-0.100 = 90.0%
For natural water of pH 6.40:
Water's pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
6.40 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
0.00 = log(mol fraction of HCO3- to H2CO3)
10^0.00 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- to H2CO3 = 1 = 50% HCO3- and 50% H2CO3
For natural water of pH 8.40:
Water's pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
8.40 pH = 6.40 + log(mol fraction of HCO3- to H2CO3)
2.00 = log(mol fraction of HCO3- to H2CO3)
10^2.00 = mol fraction of HCO3- to H2CO3
mol fraction of HCO3- to H2CO3 = 100 = 100% HCO3- and 0% H2CO3