The issue with what you describe is that is it is not how gases interact (although you do acknowledge that the gasses will mix

). Pushing a cubic meter of O2 into the bottom of a carboy/keg/whatever does not mean that you push a cubic meter of the gas that is in the top of the carboy out. For the pressure to exert on gas in the top the particles underneath must push on it. Mixing of gases in this environment is very fast. In reality the gas "pushed" out of the top of the container is a mixture of what was in the container to begin with and what was added. The size opening in the top of the vessel has zero affect on the mixing of the gases internally (unless the opening is small enough for the internal gas to pressurize). The flow rate will have the effect of higher flow will promote faster mixing of the gasses but offset diffusion from outside of the container.
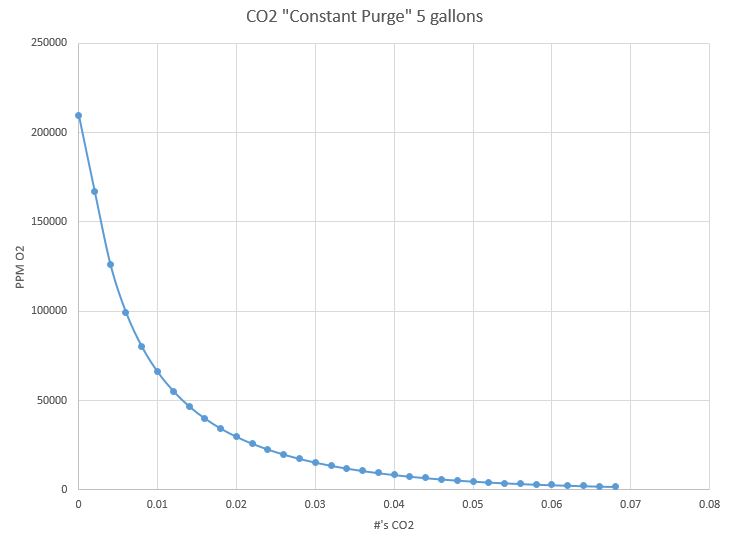
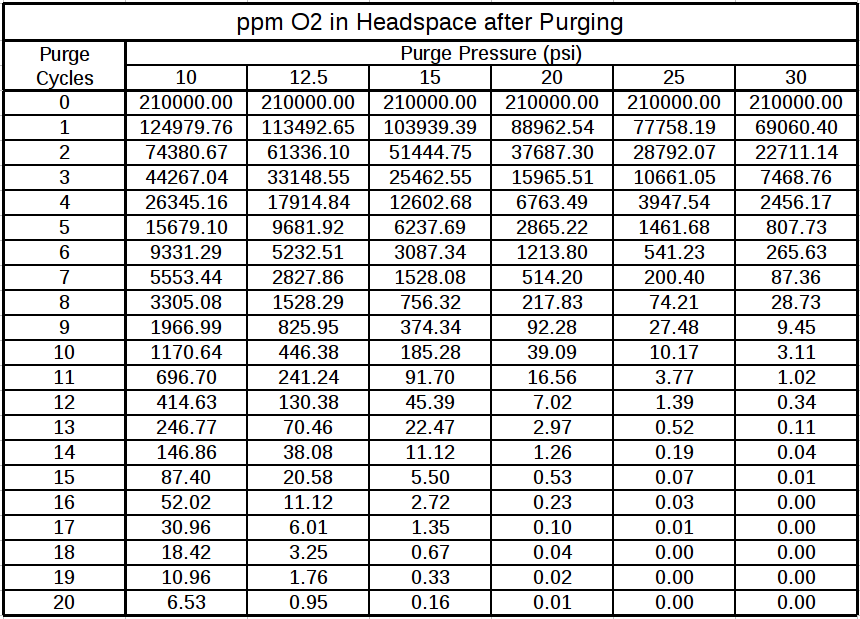
Quick math on a 5 gallon container with the assumptions of below shows the amount of #'s CO2 and PPM of O2 remaining with a "constant" flush. If you want to ignore all technical publications/books I have found or say that you drink your beer before oxidation effects take place then do so.... however, it can not be said that methods other than vacuum/pressure combination or positive displacement of liquids reduces concentration of 02 to a the range recommended by all technical sources. One of these days I'm going to find a decent dissolved O2 meter for cheap and get some empirical results.
Many people do ignore O2, many people don't control fermentation temp, many people ignore yeast counts, many people do lots of things and still make decent to good beer.
1.standard atmospheric conditions
2.mean free paths are not calculated, gases are assume to mix (this will make the calc slightly conservative)
3.Simplified to multiple small steps to eliminate integration
4.flow rate effects are not considered
5.pressurization of the vessel due to inlet vs discharge flow rates are not considered


















































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)










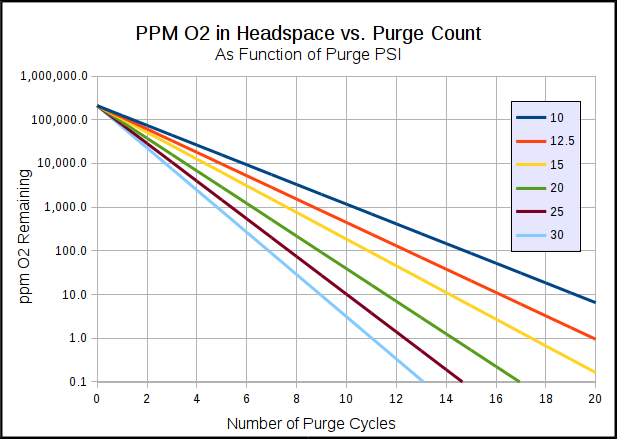
 as if other process are good it's as stable as you can get. (great if you, like me have 8+ taps)
as if other process are good it's as stable as you can get. (great if you, like me have 8+ taps)