kingschiff
Well-Known Member
- Joined
- Oct 20, 2018
- Messages
- 99
- Reaction score
- 0
See thread below for the whole convo, one member said this may be a good place to post to get answers.
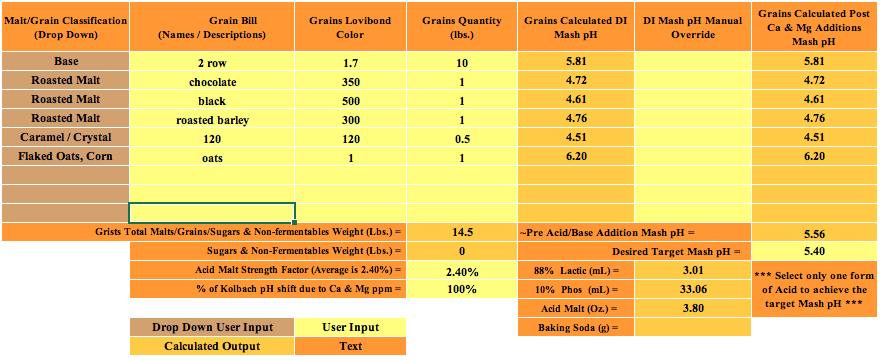
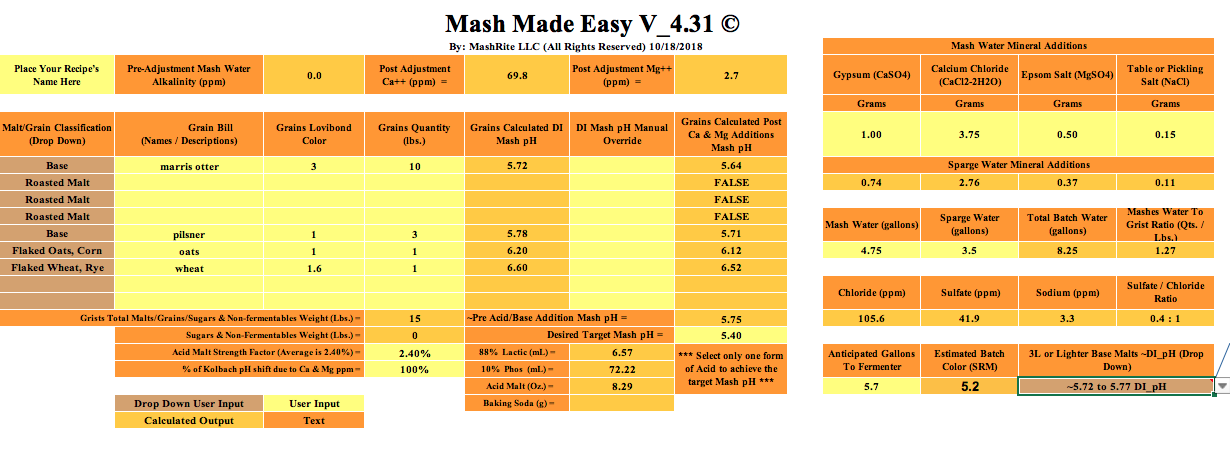
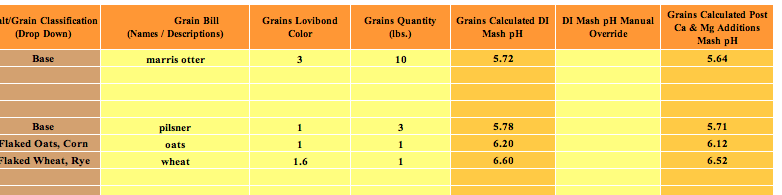
Initial question: I've been using EZwater, after missing some PHs, I did some research and found BruN water. Long story short, same specs added into both sheets. 100% distilled water, no lactic acid. EZ is giving me 5.4 mash PH, BruN water is giving me 4.18.
I have double checked #s and additions in both programs numerous times.
What gives?
https://www.homebrewtalk.com/forum/threads/ezwater-vs-brun-water-help.657252/#post-8421976
Initial question: I've been using EZwater, after missing some PHs, I did some research and found BruN water. Long story short, same specs added into both sheets. 100% distilled water, no lactic acid. EZ is giving me 5.4 mash PH, BruN water is giving me 4.18.
I have double checked #s and additions in both programs numerous times.
What gives?
https://www.homebrewtalk.com/forum/threads/ezwater-vs-brun-water-help.657252/#post-8421976


![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)