From the AMS data sheet linked in No. 10:
35ml of AMS per hl of this water reduces the alkalinity by 64 mg/litre (ppm) and increases chloride levels by 22.5 mg/litre (ppm) and sulphate levels by 31 mg/litre (ppm).
Knowing this information you can calculate the amount of AMS needed to reduce your alkalinity to the ideal level.
Yes we can because this is sufficient information to tell us what's in the product.
The chloride in 1 L of water is augmented by 22.5 mg/L which is 22.5/35.45 = 0.6347 mEq/L. The sulfate is augmented by 31 mg/L which is 31/48 = 0.6458 mEq/L. As both hydrochloric and sulfuric acid are strong with respect to mash pH we assume that the chloride and sulfate come from, respectively, those acids which would contribute hydrogen ions in quantity equal to the sum of the anion augmentation or 1.2805 mEq. They state that alkainity would be reduced by 64 mg/L which we assume to mean mg/L as CaCO3 in which case that means that 64/50 = 1.28 mEq/L protons have been supplied. As this is equal to the sum of the protons indicated by the chloride and sulfate increases we conclude that AMS is an equi
normal (WRT mash pH) mix of hydrochloric and sulfuric acids. This is the same conclusion we had previously drawn regarding this product but from Murphy's tables and not this data sheet.
As the statment regards a hectolitre treated with 35 mL of the product we conclude that the hydrochloric normality, equal to the molarity, is about 100*0.6347/35 = 1.81 N (M) and that the sulfuric acid component is about 100*0.6458/35 = 1.85 N. As sulfuric acid is diprotic the molarity is half this. The normality of the solution tells us how much we need to use to handle a particular water. It is 100*1.2805/35 = 3.66 N.
Check: To eliminate 64 ppm alkalinity (1.28 mEq/L) from an hL of water we would need 100*1.28/3.66 = 34.97 mL.
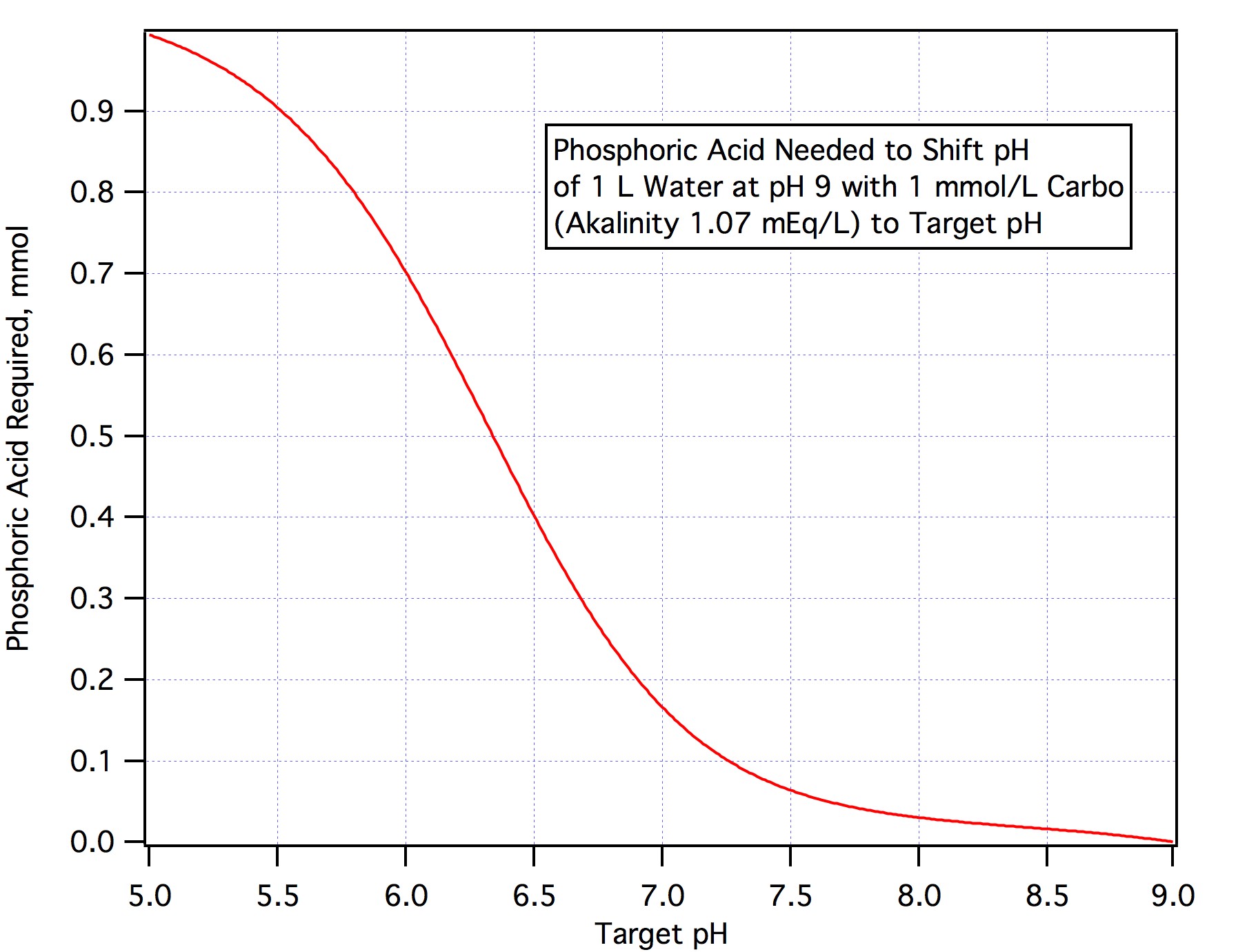
In fact we don't need to eliminate all the alkalinity but only about 90% of it (this is because alkalinity is measured to pH 4.5 and we only need to get to mash pH) thus is we had a hL of water of alkalinity 64 ppm as CaCO3 we would only need 0.9*100*1.28/3.66 = 31.5 mL.
In case this isn't clear to determine how much AMS to add to treat carbonaceous water:
1)Convert alkalinity in ppm to mEq/L by dividing by 50 (multiply by 2 and shift the decimal two to the left)
2)Compute 90% of this and multiply by the volume (L) of water you are treating
3)Divide by 3.66
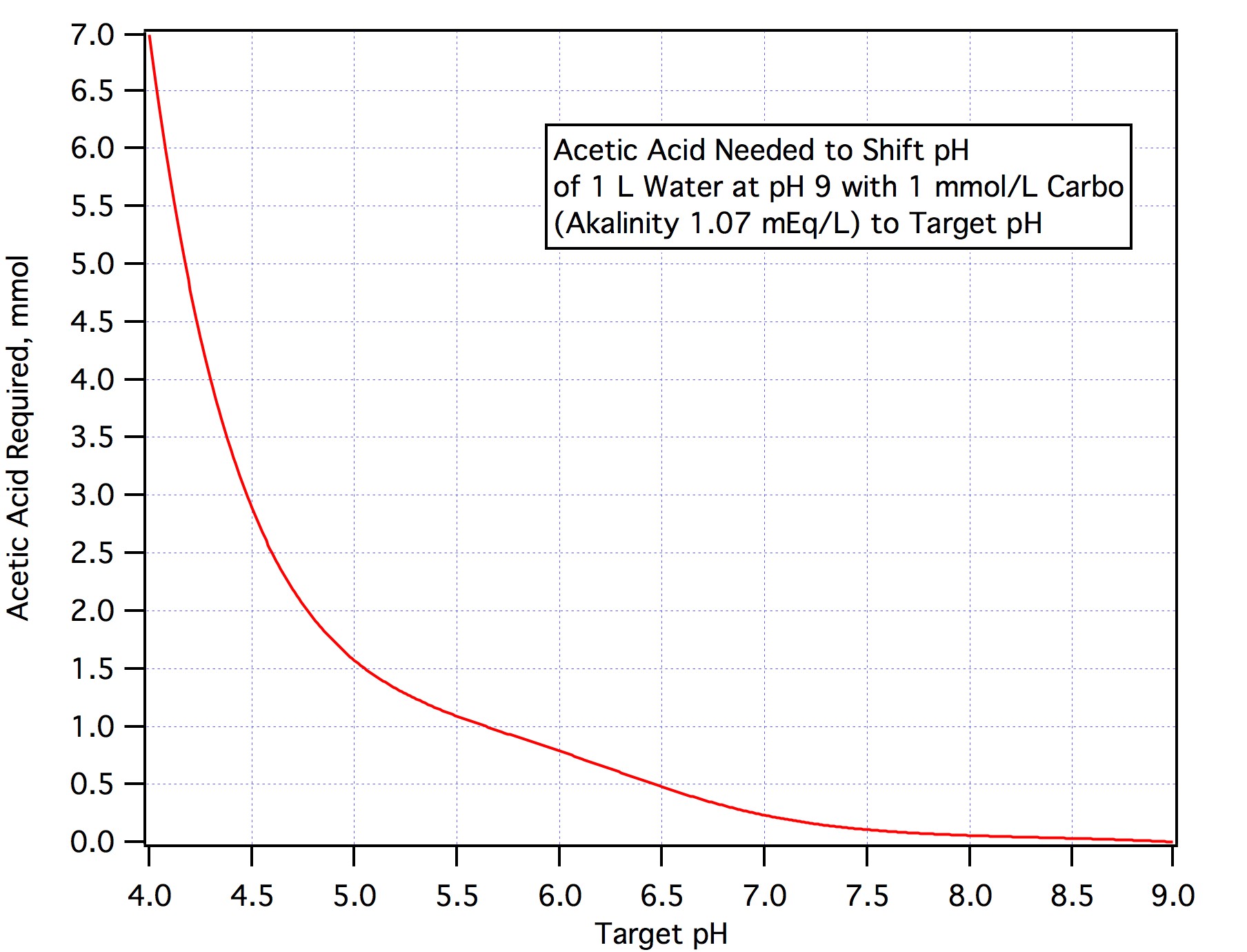
The answer from step 2 is the acid requirement in mEq/L. If you are using any other acid divide by the normality of that acid rather than the normality of AMS is step 3.
If you want to neutralize an amount of alkalinity other than 90% just convert the amount you want to nuetralize to mEq/L, multiply by the number of L and divide by 3.66.
Also from their spec sheet:
COMPOSITION An acidic aqueous solution of mineral salts
If there are indeed any additional salts in the product they are clearly not chlorides or sulfates which leaves ???
AMS adjusts liquor alkalinity without the need for boiling by removing unwanted carbonate ions and adding desirable ions, such as chloride and
sulphate in the correct ratios, ideal for most beer styles.
Apparently they have decided that the correct ratio is 22:31::Cl:SO4 which just happens to be the ratio attained when the mix is equinormal in the two acids. Coincidence?








































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)