I don't think I'd ever try to match the typical burton profile. 28 grams of gypsum? Geez. I'd stick with a Calcium under 200 and Sulfate under 400. The prediction of mash pH is realized in the 'ideal for xx SRM". If your target recipe SRM is 15 and the sheet is saying "ideal for 6-8", your mash might go too acidic. You bring up the RA/SRM range by adding baking soda if you've already got enough chloride.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Water Modification Videos, TH's Spreadsheet
- Thread starter Bobby_M
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Now you see why I am posting. Any online locations for some more reasonable water profiles for some of the basic ales and lagers?
I've got one of Ed's Haus Pale Ales and two AHS American IPAs to brew up and I'd like to try my hand at water adjustment. I've done the Pale Ale before and there is something just slightly off and I am guessing it may be the hops are a bit muddy from my unadulterated water profile. And based on my experience with that hoppy IPA tasting very muddy but bitter still, I need to adjust for that too.
I've got Mosher's Pale Ale and I think the adjustments seem reasonable for that. But a decent IPA profile is what I need now.
Thanks Bobby M and TH
I've got one of Ed's Haus Pale Ales and two AHS American IPAs to brew up and I'd like to try my hand at water adjustment. I've done the Pale Ale before and there is something just slightly off and I am guessing it may be the hops are a bit muddy from my unadulterated water profile. And based on my experience with that hoppy IPA tasting very muddy but bitter still, I need to adjust for that too.
I've got Mosher's Pale Ale and I think the adjustments seem reasonable for that. But a decent IPA profile is what I need now.
Thanks Bobby M and TH
I look at Mosher's pale ale profile as applicable to any hop-forward beer whether it's APA, Bitters, IPA, India Browns, hoppy ambers, etc.
Thanks, then I could go with the first adjustment for both recipes.
Icebrewer
Member
- Joined
- Mar 24, 2009
- Messages
- 16
- Reaction score
- 1
hey guys
My water is very low in minerals according to the local water report. The report does not include alkalinity as CaCO3 or bicarbonates.
my water profile:
Ca 2.6 mg/l
Mg 0.92 mg/l
Na 7.3 mg/l
Chloride 10.9 mg/l
Sulphate 1.7 mg/l
pH 8,5
So I figured that I could find out my water hardness as CaO according to this german water hardness equation: °1dH=(Ca(mg/l)x2,497+Mg(mg/l)x4,116)/17,9 = 0.57 °dH (CaO 10mg/l) which equals 10.53 CaCO3 mg/l according to http://www.cactus2000.de/uk/unit/masswas.shtml
I was wondering if somebody can confirm if this is right or am I totally delusional?
My water is very low in minerals according to the local water report. The report does not include alkalinity as CaCO3 or bicarbonates.
my water profile:
Ca 2.6 mg/l
Mg 0.92 mg/l
Na 7.3 mg/l
Chloride 10.9 mg/l
Sulphate 1.7 mg/l
pH 8,5
So I figured that I could find out my water hardness as CaO according to this german water hardness equation: °1dH=(Ca(mg/l)x2,497+Mg(mg/l)x4,116)/17,9 = 0.57 °dH (CaO 10mg/l) which equals 10.53 CaCO3 mg/l according to http://www.cactus2000.de/uk/unit/masswas.shtml
I was wondering if somebody can confirm if this is right or am I totally delusional?
-TH-
Well-Known Member
So I just got my gypsum from AHS and on the package it says use to harden water (I need) and lower water pH (Mine is already pretty low, I could use it higher). So with those adjustment quantities I posted, should I be concerned how much lower my pH would go? I use 5.2, as long as that can still handle it, I'd be ok?
Actually, the pH of your WATER is irrelevent. It is the pH of your MASH that's important. Mash pH can be predicted using these two factors: the residual alkalinity of your water and the color of your beer (from recipe). That is why on the spreadsheet you adjust your salts to get the RA to a level that matches your beer color. This will ensure that your mash pH is in the proper range. This also eliminates the need to use 5.2 buffer. BTW Gypsum will indeed lower the pH of your mash, as evidenced by the way it affects your RA (lowering it).
Hope that helps. Let us know how your beers turn out!
If I follow your advise above to adjust just SRM, my bare water would be fine with 6-11 SRM vs 5-14 target SRM. But, the sulfate to chloride ratio is off giving me very malty at 2.05. So I want to lower that ratio towards the bitter side, this is a Pale Ale after all. I need to either lower Cloride or raise Sulfate. Additions are what we are doing so I punch in 0.5 grams of Epsom Salt and I get a ratio of 0.73 "Very Bitter" and SRM 6-11, both acceptable.
So am I done? The Calcium, Magnesium, and Sulfate deficiencies are ignored?
Starting Water (ppm):
Ca: 2
Mg: 1.1
Na: 6.5
Cl: 8.4
SO4: 4.1
CaCO3: 11
Mash / Sparge Vol (gal): 7 / 0
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 0 / 0
CaCl2: 0 / 0
MgSO4: 0.5 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 2 / 2
Mg: 3 / 3
Na: 7 / 7
Cl: 8 / 8
SO4: 11 / 11
CaCO3: 11 / 11
RA (mash only): 8 (6 to 11 SRM)
Cl to SO4 (total water): 0.73 (Bitter)
So am I done? The Calcium, Magnesium, and Sulfate deficiencies are ignored?
Starting Water (ppm):
Ca: 2
Mg: 1.1
Na: 6.5
Cl: 8.4
SO4: 4.1
CaCO3: 11
Mash / Sparge Vol (gal): 7 / 0
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 0 / 0
CaCl2: 0 / 0
MgSO4: 0.5 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 2 / 2
Mg: 3 / 3
Na: 7 / 7
Cl: 8 / 8
SO4: 11 / 11
CaCO3: 11 / 11
RA (mash only): 8 (6 to 11 SRM)
Cl to SO4 (total water): 0.73 (Bitter)
martinworswick
Well-Known Member
https://www.homebrewtalk.com/attach...all-grain-5-10-gall-centennial_hops_ratio.jpg
i get the adjusting for srm, and i think i've got the chloride/sulphate ratio- should i use something like the above graph to determine where an intended beer falls and adjust accordingly?so if its extra hoppy i want extra bitter and so on?
i get the adjusting for srm, and i think i've got the chloride/sulphate ratio- should i use something like the above graph to determine where an intended beer falls and adjust accordingly?so if its extra hoppy i want extra bitter and so on?
-TH-
Well-Known Member
If I follow your advise above to adjust just SRM, my bare water would be fine with 6-11 SRM vs 5-14 target SRM. But, the sulfate to chloride ratio is off giving me very malty at 2.05. So I want to lower that ratio towards the bitter side, this is a Pale Ale after all. I need to either lower Cloride or raise Sulfate. Additions are what we are doing so I punch in 0.5 grams of Epsom Salt and I get a ratio of 0.73 "Very Bitter" and SRM 6-11, both acceptable.
So am I done? The Calcium, Magnesium, and Sulfate deficiencies are ignored?
Starting Water (ppm):
Ca: 2
Mg: 1.1
Na: 6.5
Cl: 8.4
SO4: 4.1
CaCO3: 11
Mash / Sparge Vol (gal): 7 / 0
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 0 / 0
CaCl2: 0 / 0
MgSO4: 0.5 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 2 / 2
Mg: 3 / 3
Na: 7 / 7
Cl: 8 / 8
SO4: 11 / 11
CaCO3: 11 / 11
RA (mash only): 8 (6 to 11 SRM)
Cl to SO4 (total water): 0.73 (Bitter)
Ideally you want to: A) Match your RA to your SRM, B) Get your Cl to SO4 ratio to match your style, and C) Get your individual mineral levels (Ca, etc.) to within recommended ranges.
Playing with your numbers for about 3 minutes I got this:
Starting Water (ppm):
Ca: 2
Mg: 1.1
Na: 6.5
Cl: 8.4
SO4: 4.1
CaCO3: 11
Mash / Sparge Vol (gal): 7 / 0
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 2 / 0
CaSO4: 1 / 0
CaCl2: 2 / 0
MgSO4: 3 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 61 / 61
Mg: 12 / 12
Na: 7 / 7
Cl: 45 / 45
SO4: 69 / 69
CaCO3: 56 / 56
RA (mash only): 5 (6 to 10 SRM)
Cl to SO4 (total water): 0.65 (Bitter)
-TH-
Well-Known Member
hey guys
My water is very low in minerals according to the local water report. The report does not include alkalinity as CaCO3 or bicarbonates.
my water profile:
Ca 2.6 mg/l
Mg 0.92 mg/l
Na 7.3 mg/l
Chloride 10.9 mg/l
Sulphate 1.7 mg/l
pH 8,5
So I figured that I could find out my water hardness as CaO according to this german water hardness equation: °1dH=(Ca(mg/l)x2,497+Mg(mg/l)x4,116)/17,9 = 0.57 °dH (CaO 10mg/l) which equals 10.53 CaCO3 mg/l according to http://www.cactus2000.de/uk/unit/masswas.shtml
I was wondering if somebody can confirm if this is right or am I totally delusional?
Hardness as CaCO3 and Alkalinity as CaCO3 are different. For an explanation, go here and scroll about 2/3 down:
http://www.howtobrew.com/section3/chapter15-1.html
Palmers says you should be able to call your water dept. and ask for an engineer and they should have either Alkalinity as CaCO3 or Bicarbonate HCO3.
Ok, so adjust to the "Recommended Range" values. I was trying to match the Mosher's profile and it all got wonky. I got negative RA and ridiculous quantities of salts to add.
Thanks for your time and experience TH.
Thanks for your time and experience TH.
-TH-
Well-Known Member
When I use the web version, it works fine. But when I use the Open Office version, I also have the same issue. When I use the "alkalinity" as CaCO3, it gives me a very strange (wrong) value for RA. The web version gives me a RA of 151. But the spreadsheet in open office gives me a RA of -56 and "error" in the alkalinity box in results.
I now have an OpenOffice version on the website that works: www.ezwatercalculator.com
- Joined
- Aug 26, 2009
- Messages
- 284
- Reaction score
- 25
Excellent work Bobby M and -TH-.
If this does not make sense I'm blaming it on the Percocet and tying with my left hand.
I have a few questions.
Is it important for me to adjust my water for doing extract brews with or without steeping grains?
So far I have just used RO water and put in a brewing salt package from my LHBS.
I'm assuming my water for doing a partial mash should be modified?
I did a google search for Brewater and tried down loading it. I think the message said it does not support 64 bit. Does that sound right?
Is there anything I can do to make it work?
Thanks..
from
"The one arm brewer"
If this does not make sense I'm blaming it on the Percocet and tying with my left hand.
I have a few questions.
Is it important for me to adjust my water for doing extract brews with or without steeping grains?
So far I have just used RO water and put in a brewing salt package from my LHBS.
I'm assuming my water for doing a partial mash should be modified?
I did a google search for Brewater and tried down loading it. I think the message said it does not support 64 bit. Does that sound right?
Is there anything I can do to make it work?
Thanks..
from
"The one arm brewer"
You can get away with using straight RO for extract brews. I'd definitely build up the profile for partial mash, especially if you get more than half the fermentables from grain.
- Joined
- Aug 26, 2009
- Messages
- 284
- Reaction score
- 25
Thanks for the reply.
SmallFloorBrewing
Member
- Joined
- Oct 8, 2009
- Messages
- 18
- Reaction score
- 0
Actually, the pH of your WATER is irrelevent. It is the pH of your MASH that's important. Mash pH can be predicted using these two factors: the residual alkalinity of your water and the color of your beer (from recipe). That is why on the spreadsheet you adjust your salts to get the RA to a level that matches your beer color. This will ensure that your mash pH is in the proper range. This also eliminates the need to use 5.2 buffer. BTW Gypsum will indeed lower the pH of your mash, as evidenced by the way it affects your RA (lowering it).
Hope that helps. Let us know how your beers turn out!
So does this mean that, if I am brewing a 5.4 SRM beer, and my residual alkalinity is good for beers between 4-7 SRM (according to the spreadsheet), that my pH is within range?
I'd still be tempted to use the 5.2 just in case the spreadsheet isn't accurate enough.
I wouldn't craft the water profile and use 5.2 at the same time. One or the other. Ever since I started using this spreadsheet (which is based completely on Palmer's data), my mash pH has measured in suitable range.
Doing my first brew with a water adjustment today. Thanks Bobby M and TH. I was surprised at how small the quantities were physically. Grams is small! The 1# packages should last me quite some time.
2 chalk
1 gypsum
2 Calcium Chrloride
3 Epsom Salt
As you suggested TH. The RA fits the SRM and it listed as bitter which is good since this is an IPA. Thanks again!
2 chalk
1 gypsum
2 Calcium Chrloride
3 Epsom Salt
As you suggested TH. The RA fits the SRM and it listed as bitter which is good since this is an IPA. Thanks again!
Great write up Bobby, I just have a couple questions as im still a nub trying to get into water.
1) I saw an earlier post in this thread.. pH 5.2 stabilizer isnt needed at all correct? I mean as long as the beer SRM matches the range in the alkalinity.
2) I run my water through a 0.5 micron carbon filter before I use it for brewing. What effect does that have on the other minerals? I use it specifically because I had an issue with chloromine once and it cant boil out. (plus my water tastes like PVC pipe).
1) I saw an earlier post in this thread.. pH 5.2 stabilizer isnt needed at all correct? I mean as long as the beer SRM matches the range in the alkalinity.
2) I run my water through a 0.5 micron carbon filter before I use it for brewing. What effect does that have on the other minerals? I use it specifically because I had an issue with chloromine once and it cant boil out. (plus my water tastes like PVC pipe).
A buddy of mine had both pre and post filter water tested the the Ca dropped by 3ppm and the Total Alkalinity dropped from 82 to 72ppm. For the most part, the filter does nothing to dissolved minerals.
GreenMonti
Well-Known Member
- Joined
- Nov 29, 2009
- Messages
- 1,268
- Reaction score
- 67
Nice work Bobby_M. Thank you for putting this together. I am just getting interested in water profiles. This helps out a lot.
This is just a thought.
I know that municiple water is different at times. What you get depends on what they were supplying that day. I don't know how different the water is or what it is that they change. Does anybody know what I am talking about? If so, are the changes big enough to even matter?
Sorry if this was asked or adressed eairlier on. I didn't read the whole thing.
This is just a thought.
I know that municiple water is different at times. What you get depends on what they were supplying that day. I don't know how different the water is or what it is that they change. Does anybody know what I am talking about? If so, are the changes big enough to even matter?
Sorry if this was asked or adressed eairlier on. I didn't read the whole thing.
I know that my water system remained within a few ppm on everything between two tests 3 months apart but I'm hoping to talk another person on my system into sending a sample in February.
kal
Well-Known Member
Love the spreadsheet TH! Phenominal work. Good job. It's the first time someone's actually explained residual alkalinity as a function of SRM to try and get your mash into the right pH range (5.1-5.3). Good job!
A question/comment however:
I understand how and why salts are added to the mash and boil per the spreadsheet but I'm worried that when I slowly rinse my grain bed during fly sparging over 60 minutes with this untreated pH 9.3 water at 168F that I may leech tannins. When sparging I can see the untreated clear sparge water pushing down the sweet wort. So am I not pushing a pH 9.3 solution past grains? Can't this leech tannins? Shouldn't this sparge water be treated to ensure it's under a pH of 6?
I guess my question is: Should I acidify my sparge water (and only my sparge water) to bring it down to around 6 pH or so to avoid issues? If yes, how does this affect my boil kettle additions of salts using this spreadsheet? Using acid to lower the pH will then work with other salts and possibly lower the pH of the sweet wort collected in the brew kettle to something too low, no?
I have a pH meter now and will be measuring pH throughout the process to check/confirm this but would love to hear from some experienced people first. Thanks!
Kal
A question/comment however:
Understood, but what what slow fly sparging however? My city's water is soft with a high pH of 9.3 (confirmed with my pH meter).Actually, the pH of your WATER is irrelevent. It is the pH of your MASH that's important.
I understand how and why salts are added to the mash and boil per the spreadsheet but I'm worried that when I slowly rinse my grain bed during fly sparging over 60 minutes with this untreated pH 9.3 water at 168F that I may leech tannins. When sparging I can see the untreated clear sparge water pushing down the sweet wort. So am I not pushing a pH 9.3 solution past grains? Can't this leech tannins? Shouldn't this sparge water be treated to ensure it's under a pH of 6?
I guess my question is: Should I acidify my sparge water (and only my sparge water) to bring it down to around 6 pH or so to avoid issues? If yes, how does this affect my boil kettle additions of salts using this spreadsheet? Using acid to lower the pH will then work with other salts and possibly lower the pH of the sweet wort collected in the brew kettle to something too low, no?
I have a pH meter now and will be measuring pH throughout the process to check/confirm this but would love to hear from some experienced people first. Thanks!
Kal
GreenMonti
Well-Known Member
- Joined
- Nov 29, 2009
- Messages
- 1,268
- Reaction score
- 67
On the adjustment spreadsheet, is it better to use the total alk or to use the bicarbonate numbers?
kal
Well-Known Member
I'm brewing a clone of Fuller's London Pride soon. They say they Burtonize the water but like BobbyM I find the Burton numbers bit over the top so I'm going with Moser's Ideal Pale Ale numbers.
My beer's specs: 1.044 SG, 11 SRM, 30 IBU
What I plan on doing to my soft Ottawa water:
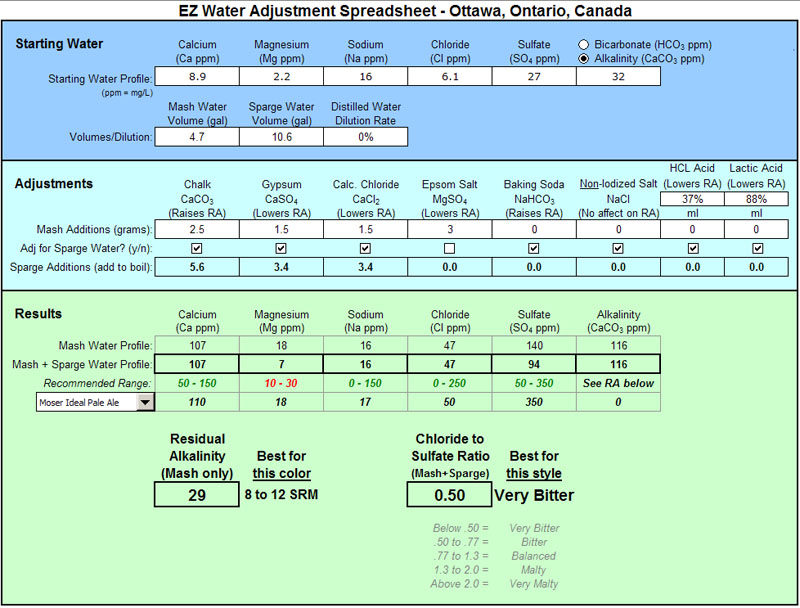
Note that my Sulfate amount in pretty low compared to Mosers (140 in the mash and 94 in the final product, instead of 350 in both). This because I want something not overly bitter so I want to keep the Chloride to Sulfate ratio somewhat reasonable (1:2). I'm also leaving out the Epson salt (MgS04) out of the boil kettle to do that, but get the mash into the right pH range.
This is the first time I'm doing anything to treat my water so comments are appreciated!
Kal
My beer's specs: 1.044 SG, 11 SRM, 30 IBU
What I plan on doing to my soft Ottawa water:

Note that my Sulfate amount in pretty low compared to Mosers (140 in the mash and 94 in the final product, instead of 350 in both). This because I want something not overly bitter so I want to keep the Chloride to Sulfate ratio somewhat reasonable (1:2). I'm also leaving out the Epson salt (MgS04) out of the boil kettle to do that, but get the mash into the right pH range.
This is the first time I'm doing anything to treat my water so comments are appreciated!
Kal
harpo
Well-Known Member
OK, so I am reading this entire thread and doing lots of goofing around with the spreadsheet. BobbyM, you mentioned in your videos about not including the additions to the boil of some of the sodium (If I remember correctly). Which ones are candidates to leave out of the sparge+mash profile (add to boil)? Is there any reason to do this? Does it affect the RA? I see that gypsum, epsom and table salt change the chloride to sulfate ratio, but does it affect the RA at all? Maybe a dumb question...
I just asked my city water dept about my pH level from the tap our water has very low ionic strength due to a low amount of dissolved minerals. They told me that over the past 9 years, over 300 samples had been taken and an average for the pH is 7.3 to 7.8.
Then I got confused about whether or not to change my sparge water because of the pH. Do I need to do anything about this? I am rapidly heading toward brain explosion about all this...
here is the raw info, using Edinburgh as a target:
Starting Water (ppm):
Ca: 15.4
Mg: 2.7
Na: 7.5
Cl: 7.9
SO4: 22
CaCO3: 15
Mash / Sparge Vol (gal): 9 / 8.7
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 5.5 / 0
CaSO4: 2.5 / 0
CaCl2: 0 / 0
MgSO4: 5 / 0
NaHCO3: 0 / 0
NaCl: 1.5 / 1.45
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 97 / 57
Mg: 16 / 10
Na: 25 / 25
Cl: 35 / 35
SO4: 120 / 72
CaCO3: 112 / 64
RA (mash only): 33 (8 to 13 SRM)
Cl to SO4 (total water): 0.48 (Very Bitter)
Thanks.
I just asked my city water dept about my pH level from the tap our water has very low ionic strength due to a low amount of dissolved minerals. They told me that over the past 9 years, over 300 samples had been taken and an average for the pH is 7.3 to 7.8.
Then I got confused about whether or not to change my sparge water because of the pH. Do I need to do anything about this? I am rapidly heading toward brain explosion about all this...
here is the raw info, using Edinburgh as a target:
Starting Water (ppm):
Ca: 15.4
Mg: 2.7
Na: 7.5
Cl: 7.9
SO4: 22
CaCO3: 15
Mash / Sparge Vol (gal): 9 / 8.7
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 5.5 / 0
CaSO4: 2.5 / 0
CaCl2: 0 / 0
MgSO4: 5 / 0
NaHCO3: 0 / 0
NaCl: 1.5 / 1.45
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 97 / 57
Mg: 16 / 10
Na: 25 / 25
Cl: 35 / 35
SO4: 120 / 72
CaCO3: 112 / 64
RA (mash only): 33 (8 to 13 SRM)
Cl to SO4 (total water): 0.48 (Very Bitter)
Thanks.
southpawbrew
Well-Known Member
I came upon this thread to close to my next brew day to be able to absorb all the great information here. Thanks ahead to everyone that has included info and tools in this and other threads it has been tremendously helpful. I would love feedback on my water profiles that I have worked up (very quickly) with the help of this thread, bobbym's videos, and the ez calculator.
I am brewing a Red Rocket Clone, beirmuncher's Cream of 3 Crops, and a Hefeweizen. I found a need to dilute my water with different %'s of RO to bring done some high levels in my water.
I used the Burton profile for the Red Rocket Clone but didn't go quite to the high levels on some components:
Starting Water (ppm):
Ca: 43
Mg: 37
Na: 34
Cl: 29
SO4: 72
CaCO3: 230
Mash / Sparge Vol (gal): 5.31 / 12
Dilution Rate: 50%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 4 / 9.04
CaSO4: 18 / 40.68
CaCl2: 0 / 0
MgSO4: 1 / 2.26
NaHCO3: 2 / 4.52
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 305 / 305
Mg: 23 / 23
Na: 44 / 44
Cl: 15 / 15
SO4: 555 / 555
CaCO3: 306 / 306
RA (mash only): 75 (11 to 16 SRM)
Cl to SO4 (total water): 0.03 (Very Bitter)
Cream of 3 crops (Edinburgh profile, seemed balanced for the lighter SRM):
Starting Water (ppm):
Ca: 43
Mg: 37
Na: 34
Cl: 29
SO4: 72
CaCO3: 230
Mash / Sparge Vol (gal): 5.31 / 12
Dilution Rate: 60%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 6 / 0
CaCl2: 5 / 11.3
MgSO4: 3 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 153 / 106
Mg: 29 / 19
Na: 14 / 14
Cl: 132 / 132
SO4: 254 / 98
CaCO3: 92 / 92
RA (mash only): -34 (2 to 7 SRM)
Cl to SO4 (total water): 1.35 (Malty)
Hefeweizen (Munich Profile)
Starting Water (ppm):
Ca: 43
Mg: 37
Na: 34
Cl: 29
SO4: 72
CaCO3: 230
Mash / Sparge Vol (gal): 2.5 / 6
Dilution Rate: 90%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 1 / 2.4
CaSO4: 1.5 / 0
CaCl2: 2 / 0
MgSO4: 1 / 2.4
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 140 / 74
Mg: 14 / 14
Na: 3 / 3
Cl: 105 / 33
SO4: 137 / 74
CaCO3: 86 / 86
RA (mash only): -22 (3 to 8 SRM)
Cl to SO4 (total water): 0.44 (Very Bitter)
I am brewing a Red Rocket Clone, beirmuncher's Cream of 3 Crops, and a Hefeweizen. I found a need to dilute my water with different %'s of RO to bring done some high levels in my water.
I used the Burton profile for the Red Rocket Clone but didn't go quite to the high levels on some components:
Starting Water (ppm):
Ca: 43
Mg: 37
Na: 34
Cl: 29
SO4: 72
CaCO3: 230
Mash / Sparge Vol (gal): 5.31 / 12
Dilution Rate: 50%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 4 / 9.04
CaSO4: 18 / 40.68
CaCl2: 0 / 0
MgSO4: 1 / 2.26
NaHCO3: 2 / 4.52
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 305 / 305
Mg: 23 / 23
Na: 44 / 44
Cl: 15 / 15
SO4: 555 / 555
CaCO3: 306 / 306
RA (mash only): 75 (11 to 16 SRM)
Cl to SO4 (total water): 0.03 (Very Bitter)
Cream of 3 crops (Edinburgh profile, seemed balanced for the lighter SRM):
Starting Water (ppm):
Ca: 43
Mg: 37
Na: 34
Cl: 29
SO4: 72
CaCO3: 230
Mash / Sparge Vol (gal): 5.31 / 12
Dilution Rate: 60%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 6 / 0
CaCl2: 5 / 11.3
MgSO4: 3 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 153 / 106
Mg: 29 / 19
Na: 14 / 14
Cl: 132 / 132
SO4: 254 / 98
CaCO3: 92 / 92
RA (mash only): -34 (2 to 7 SRM)
Cl to SO4 (total water): 1.35 (Malty)
Hefeweizen (Munich Profile)
Starting Water (ppm):
Ca: 43
Mg: 37
Na: 34
Cl: 29
SO4: 72
CaCO3: 230
Mash / Sparge Vol (gal): 2.5 / 6
Dilution Rate: 90%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 1 / 2.4
CaSO4: 1.5 / 0
CaCl2: 2 / 0
MgSO4: 1 / 2.4
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 140 / 74
Mg: 14 / 14
Na: 3 / 3
Cl: 105 / 33
SO4: 137 / 74
CaCO3: 86 / 86
RA (mash only): -22 (3 to 8 SRM)
Cl to SO4 (total water): 0.44 (Very Bitter)
- Joined
- Apr 25, 2009
- Messages
- 962
- Reaction score
- 2
How does these water adjustments look for a English IPA? Can I add all the salts to on pot? Then separate the water for strike and sparg water. Or should I add all the salts to the the mash/strike water only? Last, is the balance too far off to very bitter?


The pic is too small to see what's going on. If you select the third worksheet, you can copy and paste the raw data as text and it's very readable.
- Joined
- Apr 25, 2009
- Messages
- 962
- Reaction score
- 2
Here is the water profile I am trying to achieve.
Target Beer Style Ca+2 Mg+2 Na+1 Cl-1 SO4-2 HCO3-1 RA Cl to SO4
Burton India Pale Ale 352 24 44 16 820 320 -3 0.02
Starting Water (ppm):
Ca: 26.4
Mg: 12.6
Na: 40
Cl: 90
SO4: 87.1
HCO3: 49
Mash / Sparge Vol (gal): 2.93 / 8
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 5 / 0
CaSO4: 8 / 0
CaCl2: 0 / 0
MgSO4: 1 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 371 / 119
Mg: 21 / 15
Na: 40 / 40
Cl: 90 / 90
SO4: 525 / 204
CaCO3: 261 / 99
RA (mash only): -16 (4 to 9 SRM)
Cl to SO4 (total water): 0.44 (Very Bitter)
Target Beer Style Ca+2 Mg+2 Na+1 Cl-1 SO4-2 HCO3-1 RA Cl to SO4
Burton India Pale Ale 352 24 44 16 820 320 -3 0.02
Starting Water (ppm):
Ca: 26.4
Mg: 12.6
Na: 40
Cl: 90
SO4: 87.1
HCO3: 49
Mash / Sparge Vol (gal): 2.93 / 8
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 5 / 0
CaSO4: 8 / 0
CaCl2: 0 / 0
MgSO4: 1 / 0
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 371 / 119
Mg: 21 / 15
Na: 40 / 40
Cl: 90 / 90
SO4: 525 / 204
CaCO3: 261 / 99
RA (mash only): -16 (4 to 9 SRM)
Cl to SO4 (total water): 0.44 (Very Bitter)
That looks good to me for any kind of hoppy pale beer though I'd chuck another gram of MgSO4 into the boil kettle to get the total magnesium up.
- Joined
- Apr 25, 2009
- Messages
- 962
- Reaction score
- 2
Okay, thanks. Should I put all the salts in the strike water? Or in the strike water and sarge water mix, then split?
They have to go into the mash tun after you'd doughed in. The reduced pH in the mash will enable the salts to dissolve. If you put them into water, they'll just sink to the bottom and coat your pot.
A buddy of mine had both pre and post filter water tested the the Ca dropped by 3ppm and the Total Alkalinity dropped from 82 to 72ppm. For the most part, the filter does nothing to dissolved minerals.
Just got my report back from Ward Labs. I sent a sample filtered through a 1 micron whole-house filter. According to your post, this should be nearly the same as non-filtered? I'm just getting into this whole other realm of brewing. I've done quite a bit of water chemistry work on lakes and ponds, measuring and only trying to change pH. But we used reagents. This is another ballgame. By the way, my water looks like this:
Na 25
K 3
Ca 8
Mg 2
CaCO3 28
Nitrate .09
Sulfate 11
Cl 15
CO3 <1
HCO3 25
Total Alkalinity (CaCO3) 20
Looks like it's "kinda" close to Pilsen, but still needs some adjustments, no?
Would a good "starting" point on the spreadsheet be to shoot for Balanced, then adjust as needed based on style? And just what is in 5.2?
It's very pilsen like, apparently similar to Seattle. I'd probably brew a Bohemian Pils every other batch if I had that water.
I'd say getting Ca up to 50 and Mg to at least 15 is bare minimum.
For a nice pale IPA, you could do something like this:
Starting Water (ppm):
Ca: 8
Mg: 2
Na: 25
Cl: 15
SO4: 11
CaCO3: 20
Mash / Sparge Vol (gal): 5 / 4
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 1 / 0.8
CaSO4: 2 / 1.6
CaCl2: 2 / 1.6
MgSO4: 2 / 1.6
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 82 / 82
Mg: 12 / 12
Na: 25 / 25
Cl: 66 / 66
SO4: 111 / 111
CaCO3: 46 / 46
RA (mash only): -20 (4 to 8 SRM)
Cl to SO4 (total water): 0.59 (Bitter)
For a Brown in the 20SRM area, you can go like this:
Starting Water (ppm):
Ca: 8
Mg: 2
Na: 25
Cl: 15
SO4: 11
CaCO3: 20
Mash / Sparge Vol (gal): 5 / 4
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 4 / 0
CaSO4: 0 / 0
CaCl2: 1 / 0.8
MgSO4: 2 / 1.6
NaHCO3: 4 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 107 / 69
Mg: 12 / 12
Na: 83 / 57
Cl: 40 / 40
SO4: 52 / 52
CaCO3: 250 / 148
RA (mash only): 166 (19 to 24 SRM)
Cl to SO4 (total water): 0.78 (Balanced)
I'd say getting Ca up to 50 and Mg to at least 15 is bare minimum.
For a nice pale IPA, you could do something like this:
Starting Water (ppm):
Ca: 8
Mg: 2
Na: 25
Cl: 15
SO4: 11
CaCO3: 20
Mash / Sparge Vol (gal): 5 / 4
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 1 / 0.8
CaSO4: 2 / 1.6
CaCl2: 2 / 1.6
MgSO4: 2 / 1.6
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 82 / 82
Mg: 12 / 12
Na: 25 / 25
Cl: 66 / 66
SO4: 111 / 111
CaCO3: 46 / 46
RA (mash only): -20 (4 to 8 SRM)
Cl to SO4 (total water): 0.59 (Bitter)
For a Brown in the 20SRM area, you can go like this:
Starting Water (ppm):
Ca: 8
Mg: 2
Na: 25
Cl: 15
SO4: 11
CaCO3: 20
Mash / Sparge Vol (gal): 5 / 4
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 4 / 0
CaSO4: 0 / 0
CaCl2: 1 / 0.8
MgSO4: 2 / 1.6
NaHCO3: 4 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 107 / 69
Mg: 12 / 12
Na: 83 / 57
Cl: 40 / 40
SO4: 52 / 52
CaCO3: 250 / 148
RA (mash only): 166 (19 to 24 SRM)
Cl to SO4 (total water): 0.78 (Balanced)
Thanks Bobby, I guess I'm fortunate that I have some lee way in adjusting. About CaCO3 in the boil....will it disolve? Is gypsum more soluble? I'm gonna have to give the spreadsheet a thorough look. And what significance is a negative RA number?
kal
Well-Known Member
Hey guys! A couple of questions:
Question 1:
My water has Mg at 2 ppm. I find it's hard to get Mg up to the 10 ppm minimum on lighter beers (helles, american lagers, etc) without blowing out S04 given that that only way to add Mg is MgS04.
So how "important" is Mg? I've been getting everything else in range but Mg has been in the 6-7 ppm range.
Question 2:
When adding your additions to the boil, does it make sense to wait until after the hot break instead of adding when you first start sparging? Reason I ask is that I do skim off a lot of the protein build-up (foop) that appears before hotbreak. I'm wondering if skimming it off ends up removing any reasonable amount of salts if you add them at the start of the boil.
Kal
Question 1:
My water has Mg at 2 ppm. I find it's hard to get Mg up to the 10 ppm minimum on lighter beers (helles, american lagers, etc) without blowing out S04 given that that only way to add Mg is MgS04.
So how "important" is Mg? I've been getting everything else in range but Mg has been in the 6-7 ppm range.
Question 2:
When adding your additions to the boil, does it make sense to wait until after the hot break instead of adding when you first start sparging? Reason I ask is that I do skim off a lot of the protein build-up (foop) that appears before hotbreak. I'm wondering if skimming it off ends up removing any reasonable amount of salts if you add them at the start of the boil.
Kal
Hey guys! A couple of questions:
Question 1:
My water has Mg at 2 ppm. I find it's hard to get Mg up to the 10 ppm minimum without blowing out S04 given that that only way to add Mg is MgS04.
So how "important" is Mg? I've been getting everything else in range but Mg has been in the 6-7 ppm range.
Question 2:
When adding your additions to the boil, does it make sense to wait until after the hot break instead of adding when you first start sparging? Reason I ask is that I do skim off a lot of the protein build-up (foop) that appears before hotbreak. I'm wondering if skimming it off ends up removing any reasonable amount of salts if you add them at the start of the boil.
Kal
Question 1. I just asked a local microbrewer about his additions and this is what he said about sulfates and Mg. The additions were based on his well water, which is kinda close to mine.
Gypsum 4.1 grams/gallon
Epsom Salts 1.5
Baking Soda 0.7
This should bring up your sulfites and enhance the bitterness. Also the yeast love magnesium…helps the growth phase in initial respiration
Question 2. I'm just spit-ballin' here, but I would think that after the minerals are in solution, skimming wouldn't remove anymore than boiling would. I think they would have more tendency to precipitate out, rather than rising .
Kal, if you list your whole profile, I can play around to see if I can get something you can work with.
It's very pilsen like, apparently similar to Seattle. I'd probably brew a Bohemian Pils every other batch if I had that water.
I'd say getting Ca up to 50 and Mg to at least 15 is bare minimum.
How do you handle sparge water additions if you do no-sparge? Can you just plug in the total pre-boil volume into the spreadsheet? Or is it essential to add some of these minerals to the boil as well as the mash?
Similar threads
- Replies
- 9
- Views
- 768
- Article
- Replies
- 12
- Views
- 2K

