eps2103
Active Member
I recently picked up the LaMotte brew lab basic kit, and I used it for the first time last week. Since I have never had any experience with these tests before, I decided I'd take a water sample at the same time as my LaMotte testing, and send it off to Ward Labs to see if agrees with the brew lab kit...and mostly it does agree with the exception of Chloride and Sodium.
Ward Labs
Sodium 49
Calcium 54
Magnesium 19
Total Hardness 214
Sulfate 33
Chloride 129
Total Alkalinity 93
LaMotte
Sodium 127 (calculated per instructions)
Calcium 52
Magnesium 17
Total Hardness 200
Sulfate 30 (rough guess at the grayscale shade between 0 and 50)
Chloride 225
Total Alkalinity 125
I'd be ok with "in the ballpark" but those two numbers are not even close to what Ward tells me. According to LaMotte I have so much sodium that I'd be looking to dilute with distilled for my next batch.
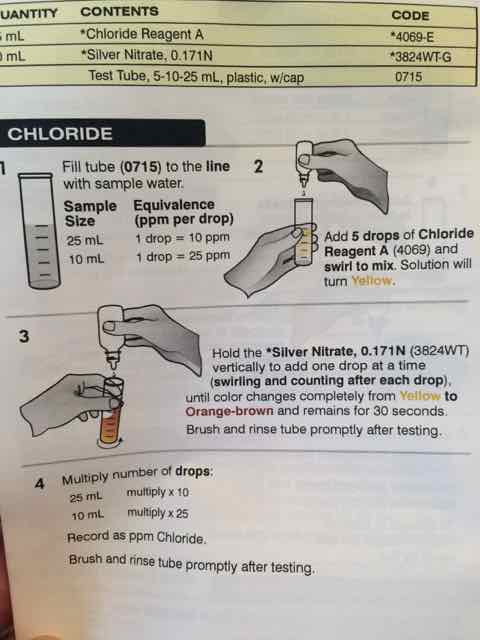
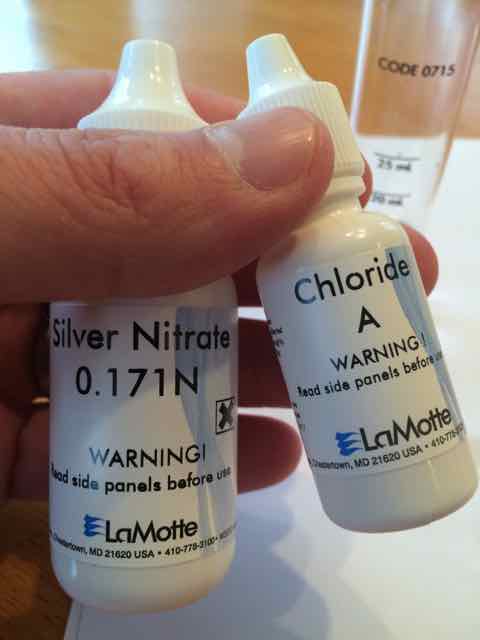
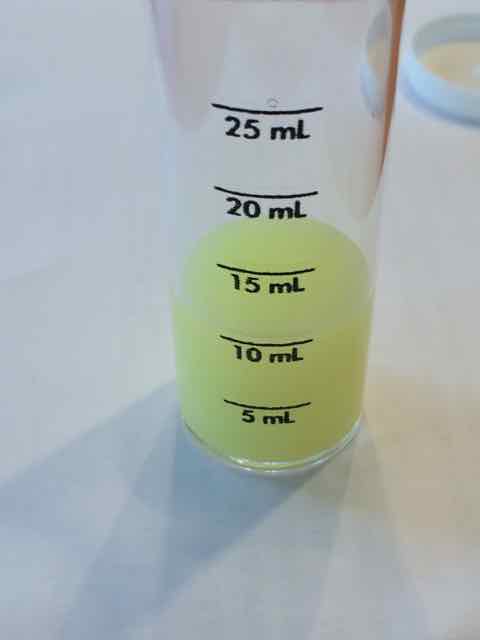
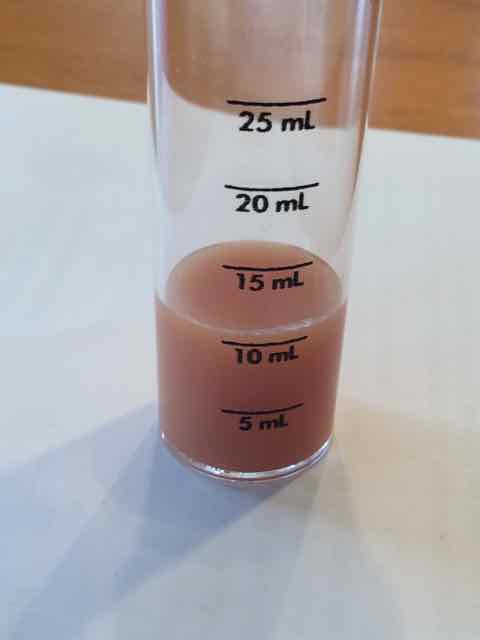
Am I missing something? I ran the Chloride test three times and I got the same results each time.
Thanks for any input...
Eric
Ward Labs
Sodium 49
Calcium 54
Magnesium 19
Total Hardness 214
Sulfate 33
Chloride 129
Total Alkalinity 93
LaMotte
Sodium 127 (calculated per instructions)
Calcium 52
Magnesium 17
Total Hardness 200
Sulfate 30 (rough guess at the grayscale shade between 0 and 50)
Chloride 225
Total Alkalinity 125
I'd be ok with "in the ballpark" but those two numbers are not even close to what Ward tells me. According to LaMotte I have so much sodium that I'd be looking to dilute with distilled for my next batch.
Am I missing something? I ran the Chloride test three times and I got the same results each time.
Thanks for any input...
Eric