- Joined
- Oct 8, 2007
- Messages
- 276
- Reaction score
- 19
I'm going to brew a Cold IPA loosely based off this recipe here: Recipe: Green Cheek It Just Works
He says the water profile he uses is: Start with carbon-filtered water that tastes good. Look at your water report and weigh out enough chloride and/or sulfate to hit a 1:1 ratio, 150 ppm of each.
With my base water profile, if I only use gypsum and CaCl to get to 1:1 at 150 my calcium ions get to almost 170. I don't have any mgSo4 on hand so my other thought was to use table salt to decrease the Ca and increase the Cl. I've never put NaCl in a beer but all I can think about is some old dude at a bar in the 70's putting some salt in a light lager. What thoughts do you all have? Here's my base water profile:
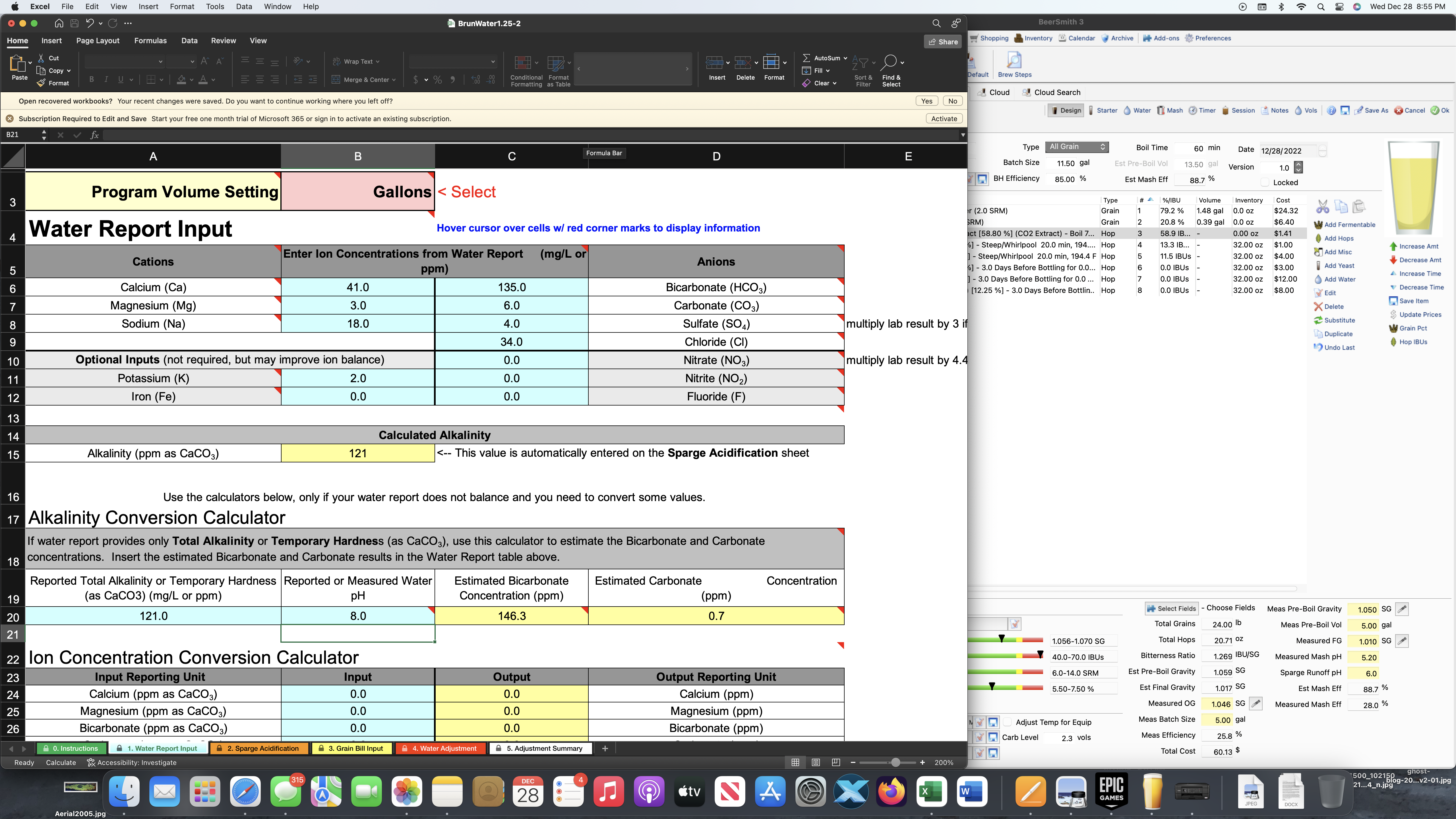
He says the water profile he uses is: Start with carbon-filtered water that tastes good. Look at your water report and weigh out enough chloride and/or sulfate to hit a 1:1 ratio, 150 ppm of each.
With my base water profile, if I only use gypsum and CaCl to get to 1:1 at 150 my calcium ions get to almost 170. I don't have any mgSo4 on hand so my other thought was to use table salt to decrease the Ca and increase the Cl. I've never put NaCl in a beer but all I can think about is some old dude at a bar in the 70's putting some salt in a light lager. What thoughts do you all have? Here's my base water profile:



