rikki_bobbi
Member
- Joined
- Mar 31, 2021
- Messages
- 5
- Reaction score
- 2
Hi all,
A relative newcomer to home brewing here with about 10 all-grain brews under my belt so far but all in the pale / bitter / IPA category despite living in Central London where the water was historically perfect for porters. I've been using brewer's friend along with tap water with a known water report to calculate my water chemistry with pale beers so far and, dialling in with acid malt in the grist, I'm getting close to my desired PH every time.
I've decided to have a crack at an India Porter loosely based on The Kernel's Export India Porter or at least Malt Miller's version of it from the grain bill they list.
I didn't want to end up making a Black IPA so I've added some Roasted Barley to the recipe (do feel free to mock me if this sounds awful) and intending to use more traditional English varieties (likely Target for bittering and Phoenix for flavour/aroma on the first batch) rather than citrus forward American varieties.
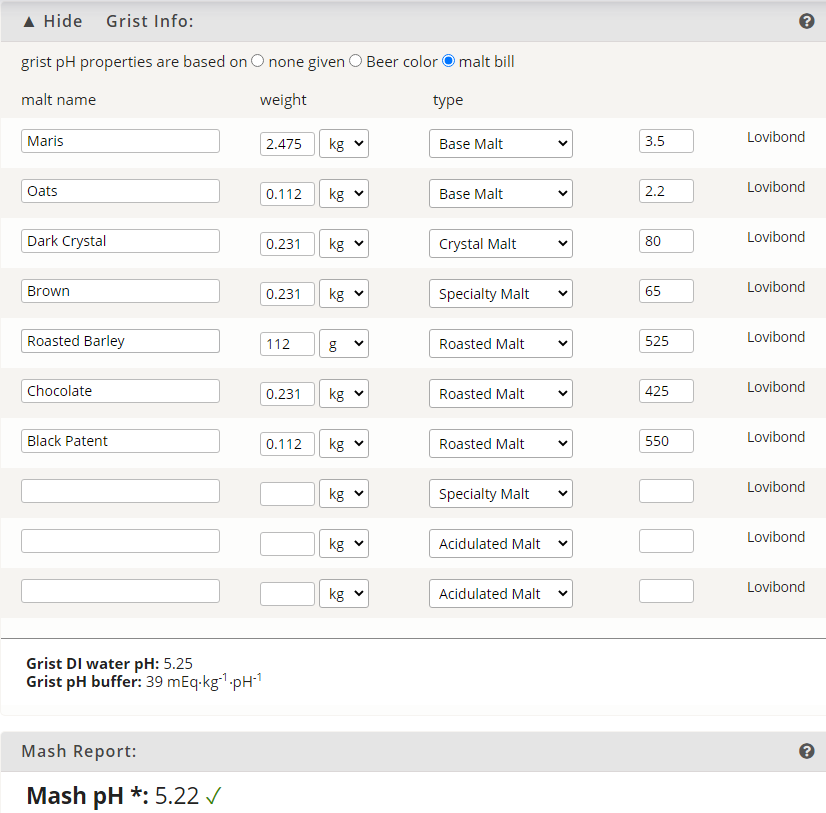
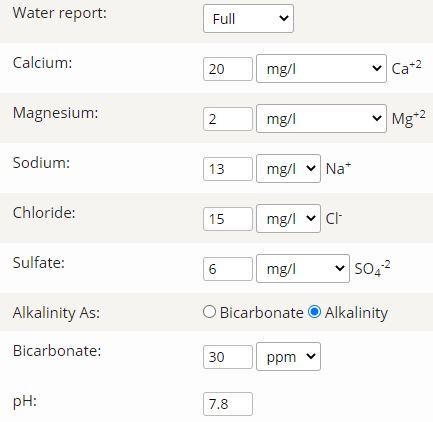
Having punched my local water report for notoriously hard water with a ph of 7.62 into Brewer's Friend's mash chemistry calculator, I'm getting a PH estimate of 3.22 based off of my grain bill!?? I know the grain bill would lower the PH a fair bit but that seems utterly ridiculous.
Water profile and grain bill are detailed here: Mash Chemistry and Brewing Water Calculator - Brewer's Friend
India Porter
12.5l Batch
OG: 1.064
IBU: 50
2.48kg Maris Otter
231g Brown Malt
231g Crystal 90
231g Chocolate
112g Roasted Barley
112g Black Patent
112g Flaked Oats
Am I going crazy?
A relative newcomer to home brewing here with about 10 all-grain brews under my belt so far but all in the pale / bitter / IPA category despite living in Central London where the water was historically perfect for porters. I've been using brewer's friend along with tap water with a known water report to calculate my water chemistry with pale beers so far and, dialling in with acid malt in the grist, I'm getting close to my desired PH every time.
I've decided to have a crack at an India Porter loosely based on The Kernel's Export India Porter or at least Malt Miller's version of it from the grain bill they list.
I didn't want to end up making a Black IPA so I've added some Roasted Barley to the recipe (do feel free to mock me if this sounds awful) and intending to use more traditional English varieties (likely Target for bittering and Phoenix for flavour/aroma on the first batch) rather than citrus forward American varieties.
Having punched my local water report for notoriously hard water with a ph of 7.62 into Brewer's Friend's mash chemistry calculator, I'm getting a PH estimate of 3.22 based off of my grain bill!?? I know the grain bill would lower the PH a fair bit but that seems utterly ridiculous.
Water profile and grain bill are detailed here: Mash Chemistry and Brewing Water Calculator - Brewer's Friend
India Porter
12.5l Batch
OG: 1.064
IBU: 50
2.48kg Maris Otter
231g Brown Malt
231g Crystal 90
231g Chocolate
112g Roasted Barley
112g Black Patent
112g Flaked Oats
Am I going crazy?