Ok let me say a few things. I am not a chemist and did not understand your explanation. What you seem to be saying is that when we add Calcium carbonate directly to the mash it releases calcium and carbonates. The latter will adsorb hydrogen and the chalk will have little if any effect on mash pH. It also reacts slowly with mash acids to have any real effect on mash parameters. It can also percolate to the kettle causing problems with pH later on. Please correct me where I am wrong I am not averse to being corrected, how else are we to learn?
It seems you did actually understand most of what I posted. Keep in mind that this is the Brewing Science forum and you are quite likely, consequently, to get blasted with raw brewing science here. If you need a fuller explanation on something just ask.
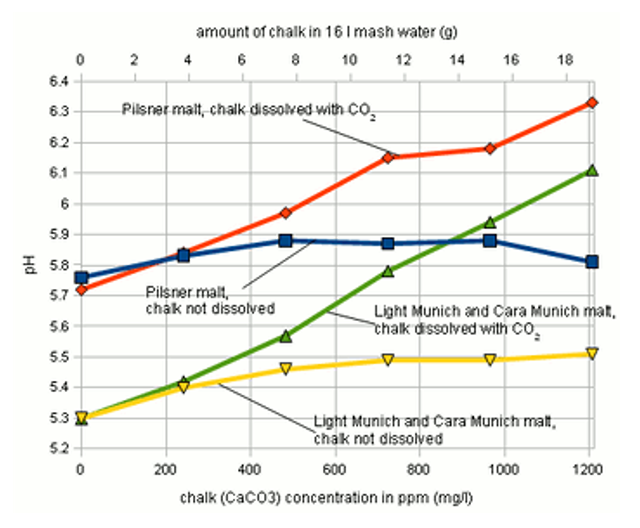
Calcium carbonate does not dissolve well. Sure but it does when put under pressure with Co2. All you need is pressurised Co2, a carbonation cap and a PET bottle. Enough Co2 will remain in solution during the mash to keep the calcium carbonate dissolved.
I really question that. As mash is hot CO2 is going to leave the solution at a faster rate than when the water is cold or room temperature. The dissolution of CaCO3 under CO2 pressure can be crudely (calcium and hydrogen ions left out) diagrammed as
CO2 + H2O --> H2CO3 --> 2HCO3- <-- +CO3-- <-- CaCO3
CO2 flows in at the left and CaCO3 flows in at the right both of which convert to bicarbonate. When pressure is relieved and especially if the solution is agitated and or heated CO2 leaves at the left end because the solution is way over saturated WRT atomospheric CO2.
CO2 + H2O <-- H2CO3 <-- 2HCO3- --> +CO3-- --> CaCO3
The CO2 flowing out of the left side is balanced by CaCO3 flowing out the right side i.e. precipitating (as microcrystals) leaving you with essentially the same situation as when you add chalk directly to the mash. The question is as to how fast the reaction takes place. So lets assume that it is very slow and that you can indeed put Ca(HCO3)2 i.e. calcium bicarbonate into your mash by dissolving chalk under CO2 pressure. This is, of course, dissociated into Ca++ and 2HCO3-. For each mmol of Ca++ which produces 1/3.5 at the kettle and more probably half that, 1/7 mEq of protons, in the mash tun you have 2 mEq of alkalinity (proton absorbing capacity). In adding calcium bicarbonate you have increased the alkalinity of your water and the pH will go up unless you add some acid to neutralize the bicarbonate. Again, you are pretty much in the same boat as with the powder. And again it is much easier to just add calcium sulfate or calcium chloride to get to the desired calcium level than it is to fiddle with the CO2 and additional acid addition.
I will also add that the main reason I add calcium carbonate to the mash when brewing dark beers like Stouts and Porters is not to provide calcium nor to balance out the mash profile but because it rounds out the caramel and roast malts providing a smooth mellowing effect.
At mash pH most of the carbonate has been converted to CO2 gas and has left the solution so that you have effectively added Ca++ ions alone and converted some malt acids to their anions thus providing the hydrogen ions necessary to convert the CO3-- to the gas. This, of course, has the effect of raising mash pH. Some feel that higher mash pH results in smoother stouts and porters. Thus the smoother effect comes from the increased alkalinity. If you need some alkalinity to keep mash pH from going to low then just add some sodium bicarbonate unless you have a real sodium phobia. That would be the only case where I could see using chalk dissolved with CO2 under pressure.
It appears to me that we can become so conditioned by technical science that we forget that beer is all about taste.
While we sometimes do push the science to the point that the art is suppressed it is always nice when the the science can explain what we see in terms of taste. That is the case here.
I don't think it any coincidence that many of the worlds finest dark beers are produced in places with relatively high concentrations of calcium carbonate in the water.

No water has a high concentration of calcium carbonate in it because calcium carbonate is not soluble in water to an appreciable extent. Surface waters tend to have about a mEq/L each (50 ppm as CaCO3) hardness and alkalinity with a pH near 8 because those are the equilibrium conditions with atmospheric partial pressure of CO2. Ground water is essentially what you proposed making with the carbonator because respiring bacteria result in subterranean CO2 pressures that are orders of magnitude higher than in the atmosphere. Thus we often see hardnesses and alkalinities of 2 mEq/L or even 3. This means that the waters have high calcium
bicarbonate content. The important fact is that they have alkalinities high enough to balance the acidities of the roast and caramel malts used. Also keep in mind that it isn't enough to know what the characteristics of a regions brewing water is. You must know what the brewery did to it, if anything, before brewing with it. Decarbonation. at least to some extent, of a highly bicarbonate water is almost guaranteed if the water is heated before mashing. Further decarbonation can be realized by heating to the boil or the addition of lime or, in a modern brewery, micro or RO filtration.