No you don't. I'm done here.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
SG and Temperature Compensation
- Thread starter Peebee
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
For the sake of us mere mortals (referencing that Wikipedia clip I posted earlier):
Density of water at 4°C ... 0.999973g/ml (Source: https://www. simetric.co.uk/si_water.htm)
Density of water sample at 20°C ... 0.998203g/ml
Therefore: S.G. of water ... 0.998203 / 0.999973 = 0.998229 ... (S.G. = 0.998)
Yeah! ... Hang on? Isn't the S.G. of water One?
... Hang on? Isn't the S.G. of water One?
Tiny differences in the numbers, but that's what it's all about! This would be the output of a "density hydrometer" or an SI standard hydrometer (configured as "20°C/4°C"). If the hydrometer was an "ordinary" one, configured as 20°C/20°C (normal for most of the world) the result would have been One!
Density of water at 4°C ... 0.999973g/ml (Source: https://www. simetric.co.uk/si_water.htm)
Density of water sample at 20°C ... 0.998203g/ml
Therefore: S.G. of water ... 0.998203 / 0.999973 = 0.998229 ... (S.G. = 0.998)
Yeah!
 ... Hang on? Isn't the S.G. of water One?
... Hang on? Isn't the S.G. of water One?Tiny differences in the numbers, but that's what it's all about! This would be the output of a "density hydrometer" or an SI standard hydrometer (configured as "20°C/4°C"). If the hydrometer was an "ordinary" one, configured as 20°C/20°C (normal for most of the world) the result would have been One!
Last edited:
I've missed a bit! Better record that, although this involves the "Full" version of my spreadsheet and I've already warned that, while displaying the S.G. directly on the weighing scales looks dead clever, it probably requires too much effort to be viable (I'll be sticking to the "Lite" version).
The "Full" version has the "standalone" capacity ... the spreadsheet is only used to calibrate and after that the SG can be read directly from the weighing scales. It only works with 100ml pyknometer bottles (it may work with 10ml bottles, but you will need very accurate, and expensive, weighing scales ... good to three decimal places of a gram, perhaps four!).
The first step is like the "Lite" version: On the "Calibration page fill in the actual (ambient) temperature that the pyknometer bottle and the sample (water) have been standing around in, then fill in the weights of the dry bottle followed by the water filled bottle.
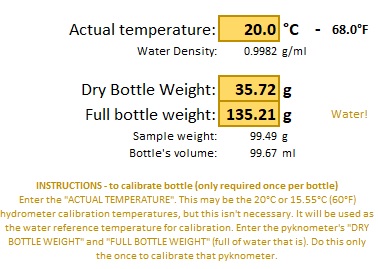
And on the same page the details that will be used to create Tare weights.
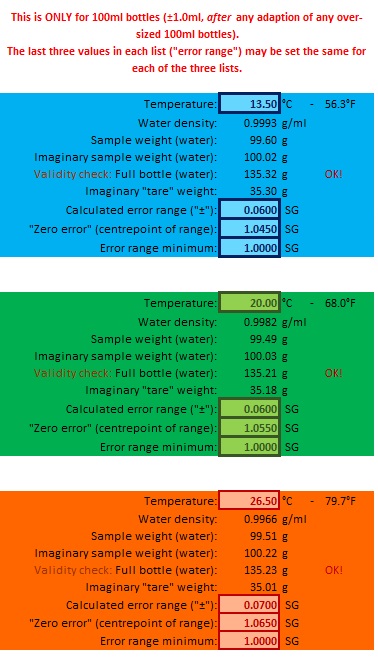
(There are some unusual parameters in this section: There were two ways of dealing with parameters for pyknometers, and I was using a "virtually adapt the bottle" method to start and switched to "actual weights" later. This section wasn't changed from "virtual" 'cos it worked, and I couldn't be bothered changing it: So, it's a bit kooky? ... Put up with it!).
What we are doing here is undoing any advantage the pyknometer has over a hydrometer and force it to operate at certain fixed temperatures. So, first off, we pick three temperatures to represent the temperature range we want to work over. Nothing too extreme, although some pyknometers are made from borosilicate glass (same as used by Pyrex), not for resistance to heat shock but to make the pyknometer dimensionally stable for very precise work (not determining the gravity of beer!). But all the same, I'm not comfortable with putting very hot water in a pyknometer bottle. For this tutorial I've picked 13.5°C, 20.0°C and 26.5°C (20.0°C would be the same temperature as a "standard" hydrometer used in much of the rest of the world).
Each temperature "configuration" has three parameters to control the error displayed in the simulation: A "zero" gravity where there would be no error (SG 1.040 is a good choice), how far either side of that point the error should be calculated (±0.060 seems good) and a minimum gravity to display an error to (1.000?). All temperature configurations may have the same error configuration, or each a different one as you please.
The result is effectively three hydrometers from the same pyknometer ... four if you count the "normal" pyknometer configuration.
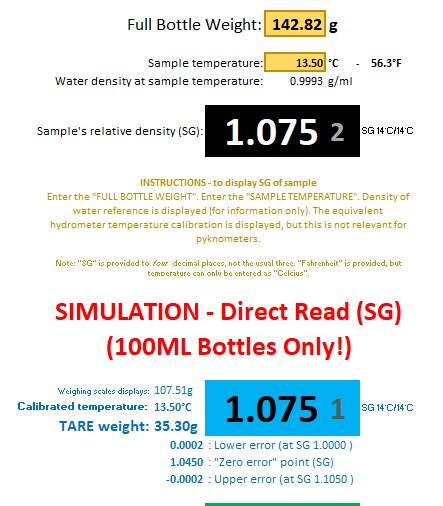
The "Simulation" page of the "Full" version allows situations to be tested. It is not required for real time operation as that is all carried out on the weighing scales. But the page does detail the size of "Tare Weights" that will be required.
The example illustrates testing with an imaginary "SG 1.075" wort. The "blue" tare weight (35.30g) is selected as being configured for a temperature closest to the imagined environment. It would induce a negligible 1/10th of a gravity point error in this example.
The "Full" version has the "standalone" capacity ... the spreadsheet is only used to calibrate and after that the SG can be read directly from the weighing scales. It only works with 100ml pyknometer bottles (it may work with 10ml bottles, but you will need very accurate, and expensive, weighing scales ... good to three decimal places of a gram, perhaps four!).
The first step is like the "Lite" version: On the "Calibration page fill in the actual (ambient) temperature that the pyknometer bottle and the sample (water) have been standing around in, then fill in the weights of the dry bottle followed by the water filled bottle.
And on the same page the details that will be used to create Tare weights.
(There are some unusual parameters in this section: There were two ways of dealing with parameters for pyknometers, and I was using a "virtually adapt the bottle" method to start and switched to "actual weights" later. This section wasn't changed from "virtual" 'cos it worked, and I couldn't be bothered changing it: So, it's a bit kooky? ... Put up with it!).
What we are doing here is undoing any advantage the pyknometer has over a hydrometer and force it to operate at certain fixed temperatures. So, first off, we pick three temperatures to represent the temperature range we want to work over. Nothing too extreme, although some pyknometers are made from borosilicate glass (same as used by Pyrex), not for resistance to heat shock but to make the pyknometer dimensionally stable for very precise work (not determining the gravity of beer!). But all the same, I'm not comfortable with putting very hot water in a pyknometer bottle. For this tutorial I've picked 13.5°C, 20.0°C and 26.5°C (20.0°C would be the same temperature as a "standard" hydrometer used in much of the rest of the world).
Each temperature "configuration" has three parameters to control the error displayed in the simulation: A "zero" gravity where there would be no error (SG 1.040 is a good choice), how far either side of that point the error should be calculated (±0.060 seems good) and a minimum gravity to display an error to (1.000?). All temperature configurations may have the same error configuration, or each a different one as you please.
The result is effectively three hydrometers from the same pyknometer ... four if you count the "normal" pyknometer configuration.
The "Simulation" page of the "Full" version allows situations to be tested. It is not required for real time operation as that is all carried out on the weighing scales. But the page does detail the size of "Tare Weights" that will be required.
The example illustrates testing with an imaginary "SG 1.075" wort. The "blue" tare weight (35.30g) is selected as being configured for a temperature closest to the imagined environment. It would induce a negligible 1/10th of a gravity point error in this example.
I'll include this bit as possibly useful too:
The "LITE" version will work with Density as well as Specific Gravity.
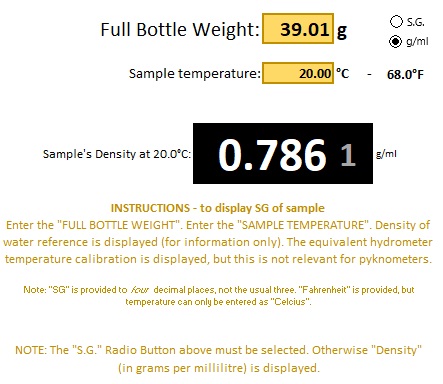
This is using a three-year-old earlier demo using isopropyl alcohol. The Density of 0.786 g/ml confirmed what I had was 99% alcohol. Remember that when working with Density (g/ml) the temperature of the sample is important (no temperature compensation like built into S.G.).
I prefer the Lite version but created the stand-alone "Full" version because people are likely to prefer its stand-alone and with "hydrometer" like workings.
(Google Sheets does not support those Radio Buttons, so in "Sheets" it looks as if nothing has been done!).
The "LITE" version will work with Density as well as Specific Gravity.
This is using a three-year-old earlier demo using isopropyl alcohol. The Density of 0.786 g/ml confirmed what I had was 99% alcohol. Remember that when working with Density (g/ml) the temperature of the sample is important (no temperature compensation like built into S.G.).
I prefer the Lite version but created the stand-alone "Full" version because people are likely to prefer its stand-alone and with "hydrometer" like workings.
(Google Sheets does not support those Radio Buttons, so in "Sheets" it looks as if nothing has been done!).
Someone woke up a very old post today (from 2009). About SG and Plato. One early post in it had:
That's a much easier way of thinking about SG! No bothering about "density". No bothering about temperature (that's a "hydrometer" thing, like I've been grizzling about above). Just weight. And we really are trying to measure differences of 0.1%, in weight, of a sample.
Weight of what? Well, you don't know precisely (from SG), but we guess "sugar" makes up the extra weight compared to plain water (and ignore any alcohol that the sugar was converted into). That "guessing" is what makes precise conversions to other measurements of extract so excruciatingly complicated (and doomed to failure). We are fortunate that in the real world we don't need anything like that precision.
It doesn't mean switching from reading an SG scale on a hydrometer to reading a Plato scale ... because the hydrometer can't tell the difference either.
... Specific gravity relates to (instead of weight of extract) the weight of the whole volume of solution relative to an equal volume of water. So, a 1.060 volume of wort is 6% heavier than the same volume of water. ...
That's a much easier way of thinking about SG! No bothering about "density". No bothering about temperature (that's a "hydrometer" thing, like I've been grizzling about above). Just weight. And we really are trying to measure differences of 0.1%, in weight, of a sample.
Weight of what? Well, you don't know precisely (from SG), but we guess "sugar" makes up the extra weight compared to plain water (and ignore any alcohol that the sugar was converted into). That "guessing" is what makes precise conversions to other measurements of extract so excruciatingly complicated (and doomed to failure). We are fortunate that in the real world we don't need anything like that precision.
It doesn't mean switching from reading an SG scale on a hydrometer to reading a Plato scale ... because the hydrometer can't tell the difference either.
Similar threads
- Replies
- 5
- Views
- 481
- Replies
- 7
- Views
- 3K

