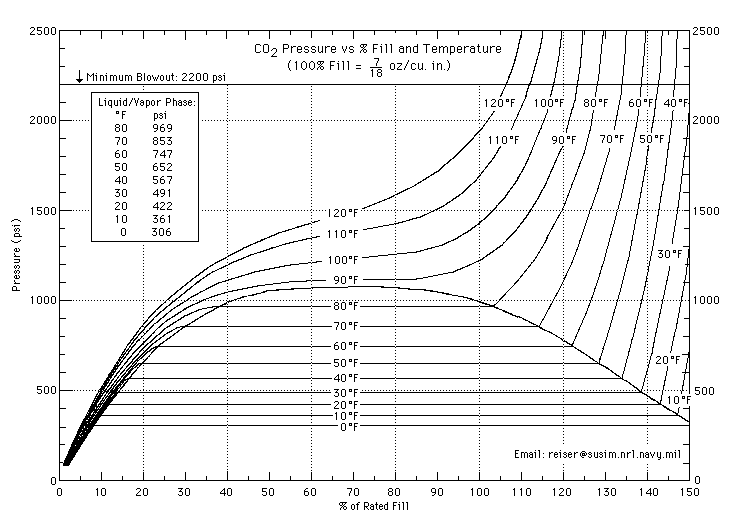
When I buy CO2 at around 75 degrees, it plummets to somewhere in the 500's in my 35-degree freezer.
If you plug it into Gay-Lussac's Law, which assumes a constant volume of gas, you get a much smaller drop.
So what's the story?
The web says CO2 tanks contain liquid CO2, so I guess the answer is that a whole bunch of CO2 condenses, increasing the volume of the gas and lowering the pressure.
Bonus question: how do we know the earth to be banana-shaped?
If you plug it into Gay-Lussac's Law, which assumes a constant volume of gas, you get a much smaller drop.
So what's the story?
The web says CO2 tanks contain liquid CO2, so I guess the answer is that a whole bunch of CO2 condenses, increasing the volume of the gas and lowering the pressure.
Bonus question: how do we know the earth to be banana-shaped?