This is in response to a request for tips on how to titrate a malt sample and determine a, b and c from the titration data. The request was that this be a Sticky. I'm not sure that is justified as I doubt many will want to undertake malt titration.
The procedure we are going to try to simplify for home brewing here is found in my paper "Predicting and Controlling Mash pH Using Simple Models for Mash Component Acid/Base Characteristics" MBAA TQ vol. 52, no. 1 2015 pp 3 - 12.
The simple model for malt is
∆Q(pHz) = a*(pHz - pHDI) + b*(pHz - pHDI)^2 + c*(pHz - pHDI)^3
∆Q(pHz) is the number of mEq of protons that must be added to a kg of malt mashed in a reasonable amount of DI water to bring the pH of the mash to pHz. If ∆Q(pHz) > 0 then one adds the protons by adding acid. If ∆Q(pHz) < 0 then protons must be removed, i.e. absorbed, by adding base.
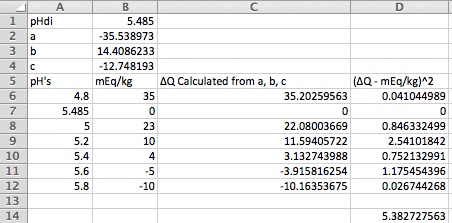
pHDI is the distilled water mash pH for this malt. a, b, and c (called a1, a2 and a3 in the paper) are coefficients which describe, along with pHDI, the malts acid/base characteristics. To determine them we add acid and base in different amounts to small portions of the malt in question mashed with DI water and measure the pH. The first pH measurement is made with no acid or base addition and the resulting pH is pHDI. We then plot the acid or base additions vs the measured pH and find values of a, b, and c which produce a curve which best fits the data.
Some example data is shown in the plot below where each circle represents a measurement. It's based on the Weyermanns pilsner malt curve in the paper but has been played around with to illustrate a few points which we'll get to. For starters, you measurements won't come out on nice whole tenths of a pH. The job is to find the curve which best fits the data in the rmse sense. We'll have Excel do that for us but first let's find out how to get the measurements.

What You Will Need
1)0.1 N Sulfuric Acid (Hach)
2)0.1 N Sodium Hydroxide (Hach)
We need to measure out protons and proton absorption capacity in mEq. An 0.1 N solution of sulfuric acid supplies 0.1 mEq/mL an an 0.1 N solution of sodium hydroxide absorbs 0.1 mEq/mL. Obviously you can get 0.1 N solutions from many places or make them up yourself if you know how and know how to standardize them. Hach sells them in 1 liter bottles and all the work is done for you but you know what the catch is - you have to pay for that.
3)Some way to measure small quantities of these solutions accurately. Syringes that can be read to tenths of a cc should do. A buret is better, an adjustable pipetter better still and a Hach Digital Titrator best (IMO)*.
4)A means to finely grind small quantities of malt. I use a Waring spice grinder.
5)A means to weigh out 50 grams of malt accurately
6)A source of DI water
7)A means to measure out 100 mL of water (great accuracy is not required here)
8)Beakers (ideally stainless steel) to hold the mini mashes (50 grams of powdered malt with 100 mL of water)
9)A good pH meter calibrated frequently
10)A means for holding the beakers at about 50° C.
11)pH 4 and 7 buffers,
Procedure:
1) Transfer small quantities of pH 4 and 7 buffer into containers which can be immersed in the water bath and place them there.
2) Measure out 100 mL DI water and place the container in the water bath at 50 °C
3) Finely grind somewhat over 50 grams of malt. Mix the grind thoroughly so the husks are evenly distributed throughout the flour. Measure 50 grams as accurately as possible and transfer to a beaker.
4) Place the beaker in the water bath (a weight will probably be necessary to keep it submerged)
5) Wait until the temperature of the DI water is 50 °C (or close to it)
6) Mix the water with the malt stirring thoroughly. Continue to stir periodically until 25 minutes have passed.
7) During the waiting period calibrate the pH meter using the 50 °C buffers.
8) Measure the pH (yes, at 50 °C). Be sure the reading is stable before accepting it (at least a couple of minutes)
9) Remove the beaker from the water bath and cool it to room temperature.
10) When the mash is at room temperature measure the pH again.
11) Look at the pHDI reading obtained at 50 °C. in the example data set it was 5.49. This is right in the middle of the mash pH range so you will be using the parameters to calculate ∆Q's at pH's above and below this value. Thus you will have to titrate with both acids and bases. It's good to have as many data points as possible, of course, but this is pretty tedious work so you might decide to shoot for measurements separated by 0.2 pH. You know that the buffering of most malts is about -40 mEq/kg•pH so that to raise the pH of a mash of this malt to 5.69 you would need to absorb something like 40*0.2 = 8 mEq/kg. You are mashing 50 gram (equals 1/20 kg) samples and so would require 8/20 = 0.4 mEq proton absorbing capacity. You have 0.1 N NaOH solution which absorbs 0.1 mEq/mL and thus would need 4 mL of that. Accurately measure 4 mL of 0.1 N NaOH into about 90 mL of DI water
12) Repeat from Steps 2 - 8 except instead of measuring out 100 mL of DI water in Step 2 make up the water NaOH mix from Step 11 to 100 mL.
From here on you continue taking data, plotting each point as it comes in, until your data set looks something like the set of circles in the picture above. To collect lower pH points use exactly the same reasoning as given in Step 11 but for acid instead of base. IOW add 4 mL of the 0.1 N H2SO4 to 90 mL of water and use that.
Obviously, as noted above, this is going to get tedious pretty quickly. About the only recommendation I have there is to see if you can handle more than one titration at a time. That way you get three points in an hour instead of only 1 but you have to figure out a protocol that lets you juggles three mashes at once. Preparing 3 malt samples and 3 water/acid/base mixes and then mixing water and malt staggered 3 - 4 minutes apart seems to work for me.
Note that your measured pH's will not be on nice even tenths of pH's nor will they be evenly spaced in 0.2 pH increments above and below pHDI. This is because, of course, the malt you are measuring won't have a = -40 mEq/kg (what a actually is is what we are trying to find out.
I'm going to stop here for fear of overflowing the size limitation. To be continued.
*This is in part because the digital titrator is accurate, easy to use, can be used for other things such as measuring alkalinity and hardness and because the NaOH titrant comes in a cartridge which is sealed in a plastic bag thus protecting it from air (CO2)
The procedure we are going to try to simplify for home brewing here is found in my paper "Predicting and Controlling Mash pH Using Simple Models for Mash Component Acid/Base Characteristics" MBAA TQ vol. 52, no. 1 2015 pp 3 - 12.
The simple model for malt is
∆Q(pHz) = a*(pHz - pHDI) + b*(pHz - pHDI)^2 + c*(pHz - pHDI)^3
∆Q(pHz) is the number of mEq of protons that must be added to a kg of malt mashed in a reasonable amount of DI water to bring the pH of the mash to pHz. If ∆Q(pHz) > 0 then one adds the protons by adding acid. If ∆Q(pHz) < 0 then protons must be removed, i.e. absorbed, by adding base.
pHDI is the distilled water mash pH for this malt. a, b, and c (called a1, a2 and a3 in the paper) are coefficients which describe, along with pHDI, the malts acid/base characteristics. To determine them we add acid and base in different amounts to small portions of the malt in question mashed with DI water and measure the pH. The first pH measurement is made with no acid or base addition and the resulting pH is pHDI. We then plot the acid or base additions vs the measured pH and find values of a, b, and c which produce a curve which best fits the data.
Some example data is shown in the plot below where each circle represents a measurement. It's based on the Weyermanns pilsner malt curve in the paper but has been played around with to illustrate a few points which we'll get to. For starters, you measurements won't come out on nice whole tenths of a pH. The job is to find the curve which best fits the data in the rmse sense. We'll have Excel do that for us but first let's find out how to get the measurements.

What You Will Need
1)0.1 N Sulfuric Acid (Hach)
2)0.1 N Sodium Hydroxide (Hach)
We need to measure out protons and proton absorption capacity in mEq. An 0.1 N solution of sulfuric acid supplies 0.1 mEq/mL an an 0.1 N solution of sodium hydroxide absorbs 0.1 mEq/mL. Obviously you can get 0.1 N solutions from many places or make them up yourself if you know how and know how to standardize them. Hach sells them in 1 liter bottles and all the work is done for you but you know what the catch is - you have to pay for that.
3)Some way to measure small quantities of these solutions accurately. Syringes that can be read to tenths of a cc should do. A buret is better, an adjustable pipetter better still and a Hach Digital Titrator best (IMO)*.
4)A means to finely grind small quantities of malt. I use a Waring spice grinder.
5)A means to weigh out 50 grams of malt accurately
6)A source of DI water
7)A means to measure out 100 mL of water (great accuracy is not required here)
8)Beakers (ideally stainless steel) to hold the mini mashes (50 grams of powdered malt with 100 mL of water)
9)A good pH meter calibrated frequently
10)A means for holding the beakers at about 50° C.
11)pH 4 and 7 buffers,
Procedure:
1) Transfer small quantities of pH 4 and 7 buffer into containers which can be immersed in the water bath and place them there.
2) Measure out 100 mL DI water and place the container in the water bath at 50 °C
3) Finely grind somewhat over 50 grams of malt. Mix the grind thoroughly so the husks are evenly distributed throughout the flour. Measure 50 grams as accurately as possible and transfer to a beaker.
4) Place the beaker in the water bath (a weight will probably be necessary to keep it submerged)
5) Wait until the temperature of the DI water is 50 °C (or close to it)
6) Mix the water with the malt stirring thoroughly. Continue to stir periodically until 25 minutes have passed.
7) During the waiting period calibrate the pH meter using the 50 °C buffers.
8) Measure the pH (yes, at 50 °C). Be sure the reading is stable before accepting it (at least a couple of minutes)
9) Remove the beaker from the water bath and cool it to room temperature.
10) When the mash is at room temperature measure the pH again.
11) Look at the pHDI reading obtained at 50 °C. in the example data set it was 5.49. This is right in the middle of the mash pH range so you will be using the parameters to calculate ∆Q's at pH's above and below this value. Thus you will have to titrate with both acids and bases. It's good to have as many data points as possible, of course, but this is pretty tedious work so you might decide to shoot for measurements separated by 0.2 pH. You know that the buffering of most malts is about -40 mEq/kg•pH so that to raise the pH of a mash of this malt to 5.69 you would need to absorb something like 40*0.2 = 8 mEq/kg. You are mashing 50 gram (equals 1/20 kg) samples and so would require 8/20 = 0.4 mEq proton absorbing capacity. You have 0.1 N NaOH solution which absorbs 0.1 mEq/mL and thus would need 4 mL of that. Accurately measure 4 mL of 0.1 N NaOH into about 90 mL of DI water
12) Repeat from Steps 2 - 8 except instead of measuring out 100 mL of DI water in Step 2 make up the water NaOH mix from Step 11 to 100 mL.
From here on you continue taking data, plotting each point as it comes in, until your data set looks something like the set of circles in the picture above. To collect lower pH points use exactly the same reasoning as given in Step 11 but for acid instead of base. IOW add 4 mL of the 0.1 N H2SO4 to 90 mL of water and use that.
Obviously, as noted above, this is going to get tedious pretty quickly. About the only recommendation I have there is to see if you can handle more than one titration at a time. That way you get three points in an hour instead of only 1 but you have to figure out a protocol that lets you juggles three mashes at once. Preparing 3 malt samples and 3 water/acid/base mixes and then mixing water and malt staggered 3 - 4 minutes apart seems to work for me.
Note that your measured pH's will not be on nice even tenths of pH's nor will they be evenly spaced in 0.2 pH increments above and below pHDI. This is because, of course, the malt you are measuring won't have a = -40 mEq/kg (what a actually is is what we are trying to find out.
I'm going to stop here for fear of overflowing the size limitation. To be continued.
*This is in part because the digital titrator is accurate, easy to use, can be used for other things such as measuring alkalinity and hardness and because the NaOH titrant comes in a cartridge which is sealed in a plastic bag thus protecting it from air (CO2)
Last edited by a moderator: