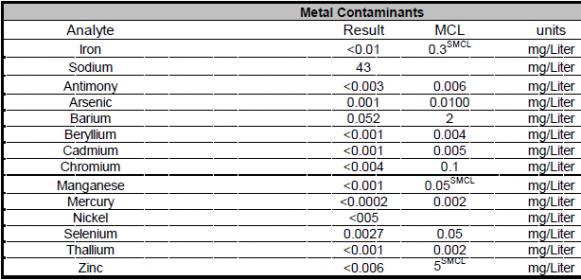
I live in Elgin, IL and the city posts a nice Water Quality report which contains (I think) everything I need to know, but I need some help to interpret (as I am no chemist).
The actual report is at: City of Elgin, Illinois - Official Website - Water Quality
Total Alkalinity - 79, pH - 8.72
---Hardness (all mg/L as CaCO3)
Total Hardness - 127
Calcium Hardness - 83
Magnesium Hardness - 42
Non-carbonate Hardness - 46
I think to get to ppm ion concentrations for Ca and Mg, I would figure the molar weight to be 40/100 (for Ca) and 24.3/100 (for Mg), or 33.3 and 10.2 respectively.
All the other important data (except bicarbonate ppm) is also in the report I think:
Ca - 33.3
Mg - 10.2
Na - 43
Cl - 112.9
SO4 - 49.8
HCO3 - ??
CO3 - ??
CaCO3 - ??
My question is this: Can I calculate the bicarbonate and carbonate levels using Total Alkalinity and pH similar to this link: HOW TO CALCULATE BICARBONATE, CARBONATE AND HYDROXIDE FROM TOTAL ALKALINITY
If so, I get HCO3 of ~92 and CO3 of ~2. What is CaCO3?
All of this data seems to jive until I plug it into Bru'N Water spreadsheet. The cations and anions are not in mass balance (there are way more anions). So it leads me to believe I am missing something.
Can anyone help me with interpretation? I like the setup of Bru'N Water's spreadsheet, but want to make sure my baseline assumptions are correct.
Also, I am planning to purchase a pH meter to validate my results, but since I already have access to this data, I want to use it as a starting point.
The actual report is at: City of Elgin, Illinois - Official Website - Water Quality
Total Alkalinity - 79, pH - 8.72
---Hardness (all mg/L as CaCO3)
Total Hardness - 127
Calcium Hardness - 83
Magnesium Hardness - 42
Non-carbonate Hardness - 46
I think to get to ppm ion concentrations for Ca and Mg, I would figure the molar weight to be 40/100 (for Ca) and 24.3/100 (for Mg), or 33.3 and 10.2 respectively.
All the other important data (except bicarbonate ppm) is also in the report I think:
Ca - 33.3
Mg - 10.2
Na - 43
Cl - 112.9
SO4 - 49.8
HCO3 - ??
CO3 - ??
CaCO3 - ??
My question is this: Can I calculate the bicarbonate and carbonate levels using Total Alkalinity and pH similar to this link: HOW TO CALCULATE BICARBONATE, CARBONATE AND HYDROXIDE FROM TOTAL ALKALINITY
If so, I get HCO3 of ~92 and CO3 of ~2. What is CaCO3?
All of this data seems to jive until I plug it into Bru'N Water spreadsheet. The cations and anions are not in mass balance (there are way more anions). So it leads me to believe I am missing something.
Can anyone help me with interpretation? I like the setup of Bru'N Water's spreadsheet, but want to make sure my baseline assumptions are correct.
Also, I am planning to purchase a pH meter to validate my results, but since I already have access to this data, I want to use it as a starting point.