Ok guys, this time I'm going to attempt to do some additions to a pale ale. That's one style that has been consistently good with my tap water, but I'm ready to try to make it great. I'll be doing a full-volume mash, biab. Attached will be screen shots from the Bru'n water spreadsheet, and links of the types of calcium chloride and calcium sulfate I'll be using.
Does it look ok? And how do I determine how much to add of each - as in, I believe the gypsum is 100% so do I just add the 3 grams of that? Then the calcium chloride is 33%, so do I divide the 2.5 grams by 33%, so I would actually add 7.8 grams (or actually ml, since it's liquid)?
https://www.bryggselv.no/råvarer/ti...id-CaCl2-33pcnt-E509-100ml-700014-p0000000743
https://www.bryggselv.no/råvarer/ti...umsulfat-CaSO4-gypsum-100g-700015-p0000000744

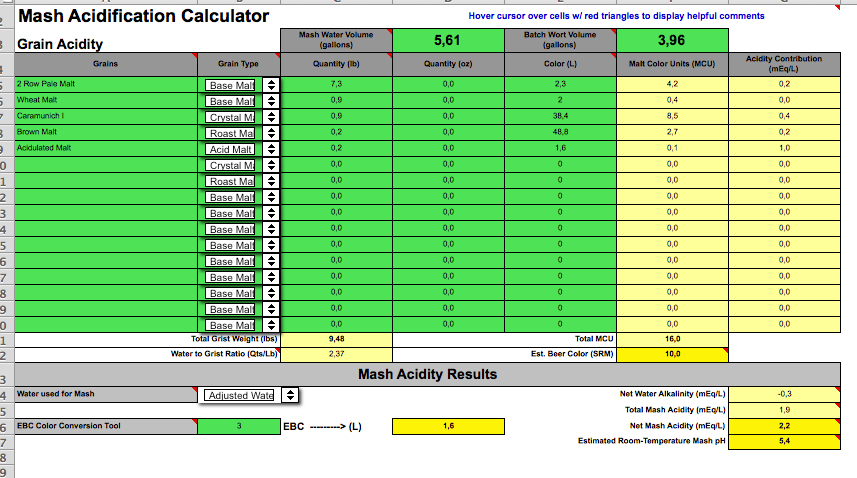
Does it look ok? And how do I determine how much to add of each - as in, I believe the gypsum is 100% so do I just add the 3 grams of that? Then the calcium chloride is 33%, so do I divide the 2.5 grams by 33%, so I would actually add 7.8 grams (or actually ml, since it's liquid)?
https://www.bryggselv.no/råvarer/ti...id-CaCl2-33pcnt-E509-100ml-700014-p0000000743
https://www.bryggselv.no/råvarer/ti...umsulfat-CaSO4-gypsum-100g-700015-p0000000744




