nudarkshadowl
Well-Known Member
- Joined
- Apr 4, 2014
- Messages
- 51
- Reaction score
- 26
So I decided on brewing the Inglewood IPA recipe from the Beersmith CCHBS AG add on for my first AG batch on my soon to be here Morebeer single tier digital sculpture and I wanted to run some things by more experienced AG brewers to make sure I am going about this in the correct way.
First off, when Ward tested my water profile it came back with the following results (I.E. Unbalanced, this was the second time they tested the water with similar results and they recommended we expand the cations tested to include NH4 or Al in order to see if it balances out.)
Please note, this was straight out of the tap, unfiltered water. I have since acquired a filtering system and plan to filter my brewing and sparge water though a 3 chamber filter directly off of my hose (RV hose line, not a garden hose) that includes a PH correction filter, a Chloramine & Hydrogen Sulfide Removal Filter, and your basic active carbon block filter. After looking into the PH correction filter, it would seem that it neutralizes acid and with my PH being base/high at 7.9, it probably wont do anything but I got the filtration system for free so I guess it doesn't matter, lol.
Heres my report:
pH 7.9
Total Dissolved Solids (TDS) Est, ppm 546
Electrical Conductivity, mmho/cm 0.91
Cations / Anions, me/L 9.4 / 10.0
ppm
Sodium, Na 90
Potassium, K 5
Calcium, Ca 67
Magnesium, Mg 24
Total Hardness, CaCO3 268
Nitrate, NO3-N 0.2 (SAFE)
Sulfate, SO4-S 73 (219ppm)
Chloride, Cl 84
Carbonate, CO3 < 1.0
Bicarbonate, HCO3 187
Total Alkalinity, CaCO3 154
Total Phosphorus, P 0.02
Total Iron, Fe < 0.01
Everything seems to be in an OK range according to EZ-Water and other articles aside from the Alkalinity/Bicarbonate being high, thus giving the 7.9 PH value. I have some permanent hardness in the water as well. I plan to bring my sparge and mash water down to 5.5 PH for this recipe and was thinking I could get there with 7ml of Lactic Acid. I have been reading Palmers Water book, and blew through about half of it today, so I'm at least learning some stuff lol.
According to EZ water, this would be bring my profile for the IPA to the following:
(Ca ppm) 67 (Mg ppm)24 (Na ppm)90 (Cl ppm)84 (SO4 ppm)219 Ratio0.38 PH5.5
When I entered in my equipment profile in Beersmith, I accounted for a 2.5 gallon deadspace volume in the MT due to the false bottom. BS game me a total water volume needed of 17.62 Gal (9.32 for the mash, and 8.30 for the sparge). See below for the recipe profile:
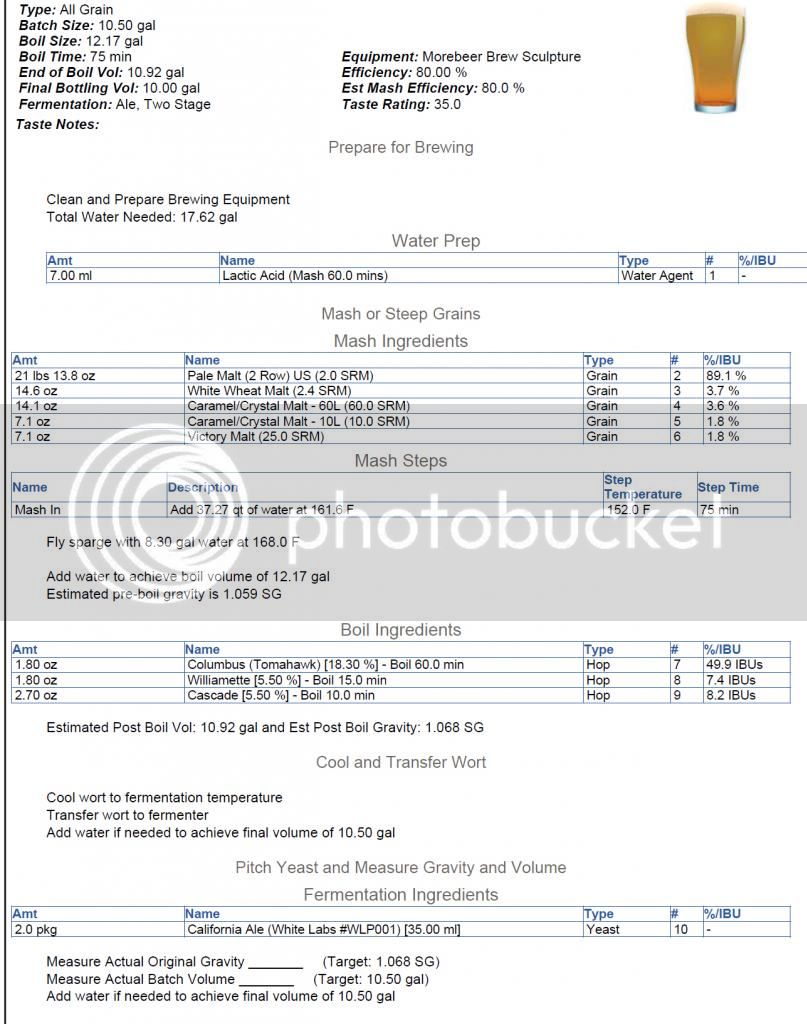
Sorry for the long winded post, but I'm just trying to make sure my figures are where they need to be. The recipe calls for 37.27 qu at 161.6 degrees for my HLT, which after doughing in will hopefully stabilize at 152 degrees for the 75 min recirc. mash, then I'll sparge the 8.30 gal at 168 degrees hopefully over a 45 min period if i'm able to figure out the flow rate balancing for the pumps.
I am probably waaaayyy over thinking this and making it more complicated that it needs to be, but I have been reading a lot on all this stuff and there is just so much information out there that I am trying to absorb as much as I can.
My biggest concern is getting my Mash/sparge PH levels to be where they need to be, while also removing any chlorine/chloramine from the water. Basically I just want to make sure my water profile is on point for my first AG run.
After reading Palmers book, I'm thinking that I should go about reducing my alkalinity in another way. Maybe with acid malt and calcium salts instead of just the lactic acid. I'm trying get good enough to just use my tap water for everything without having to cut my water with ro or distilled water. Are my sulfates too high for this style? Also, a PH meter is on the way, a Milwaukee pH Meter w/ATC from morebeer along with the storage and calibration solutions.
Cheers! And thanks again for all of the help/advice so far!
First off, when Ward tested my water profile it came back with the following results (I.E. Unbalanced, this was the second time they tested the water with similar results and they recommended we expand the cations tested to include NH4 or Al in order to see if it balances out.)
Please note, this was straight out of the tap, unfiltered water. I have since acquired a filtering system and plan to filter my brewing and sparge water though a 3 chamber filter directly off of my hose (RV hose line, not a garden hose) that includes a PH correction filter, a Chloramine & Hydrogen Sulfide Removal Filter, and your basic active carbon block filter. After looking into the PH correction filter, it would seem that it neutralizes acid and with my PH being base/high at 7.9, it probably wont do anything but I got the filtration system for free so I guess it doesn't matter, lol.
Heres my report:
pH 7.9
Total Dissolved Solids (TDS) Est, ppm 546
Electrical Conductivity, mmho/cm 0.91
Cations / Anions, me/L 9.4 / 10.0
ppm
Sodium, Na 90
Potassium, K 5
Calcium, Ca 67
Magnesium, Mg 24
Total Hardness, CaCO3 268
Nitrate, NO3-N 0.2 (SAFE)
Sulfate, SO4-S 73 (219ppm)
Chloride, Cl 84
Carbonate, CO3 < 1.0
Bicarbonate, HCO3 187
Total Alkalinity, CaCO3 154
Total Phosphorus, P 0.02
Total Iron, Fe < 0.01
Everything seems to be in an OK range according to EZ-Water and other articles aside from the Alkalinity/Bicarbonate being high, thus giving the 7.9 PH value. I have some permanent hardness in the water as well. I plan to bring my sparge and mash water down to 5.5 PH for this recipe and was thinking I could get there with 7ml of Lactic Acid. I have been reading Palmers Water book, and blew through about half of it today, so I'm at least learning some stuff lol.
According to EZ water, this would be bring my profile for the IPA to the following:
(Ca ppm) 67 (Mg ppm)24 (Na ppm)90 (Cl ppm)84 (SO4 ppm)219 Ratio0.38 PH5.5
When I entered in my equipment profile in Beersmith, I accounted for a 2.5 gallon deadspace volume in the MT due to the false bottom. BS game me a total water volume needed of 17.62 Gal (9.32 for the mash, and 8.30 for the sparge). See below for the recipe profile:

Sorry for the long winded post, but I'm just trying to make sure my figures are where they need to be. The recipe calls for 37.27 qu at 161.6 degrees for my HLT, which after doughing in will hopefully stabilize at 152 degrees for the 75 min recirc. mash, then I'll sparge the 8.30 gal at 168 degrees hopefully over a 45 min period if i'm able to figure out the flow rate balancing for the pumps.
I am probably waaaayyy over thinking this and making it more complicated that it needs to be, but I have been reading a lot on all this stuff and there is just so much information out there that I am trying to absorb as much as I can.
My biggest concern is getting my Mash/sparge PH levels to be where they need to be, while also removing any chlorine/chloramine from the water. Basically I just want to make sure my water profile is on point for my first AG run.
After reading Palmers book, I'm thinking that I should go about reducing my alkalinity in another way. Maybe with acid malt and calcium salts instead of just the lactic acid. I'm trying get good enough to just use my tap water for everything without having to cut my water with ro or distilled water. Are my sulfates too high for this style? Also, a PH meter is on the way, a Milwaukee pH Meter w/ATC from morebeer along with the storage and calibration solutions.
Cheers! And thanks again for all of the help/advice so far!



