Finally, some science to prove I am wrong... and right

And the answer is:
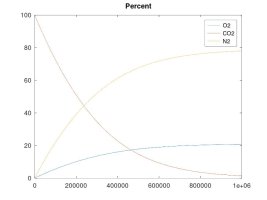
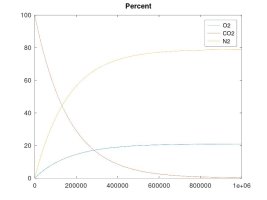
A keg that is left at ambient pressure and has a hose or gasket that is permeable to air, will EVENTUALLY* contain 21% oxygen inside.
A keg that is pressurized and maintained to 15 psi with a continuous supply of CO2, will EVENTUALLY* contain 10.5% oxygen inside (and at 40 psi, the O2 level would equalize to 7.8%)
I am offering up a reward of my treasured Apple Brandy Aged Gulden Draak, Ommegang 20th Anniversary Bourbon Barrel Aged Belgian Strong Ale, and 2018 Thomas Hardy's Ale 50th Anniversary Golden Edition to anyone that can disprove the gist of the claims in this post

Out of a desire to know the facts, I dug deeper into the science and then simulated gas behavior under varying conditions. In case anyone is interested in discerning truth vs. partial-truth vs. misinformation, I am posting the painfully lengthy results of this effort.
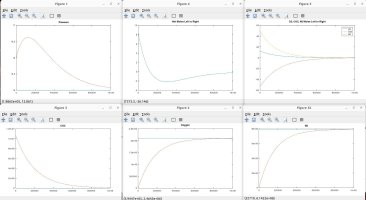
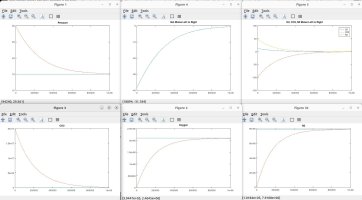
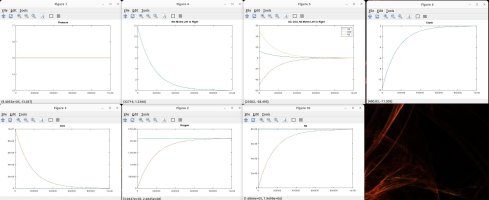
I talked with a friend who is a physicist and he clarified some things and verified some things, which inspired me to model the scenarios discussed. Using the proven laws of physics, (Boyle's, Dalton's, Avogadro's, Amonton's, Charles', Graham's, kinetic molecular laws, laws of thermodynamics, laws of motion), I generated several Matlab models to simulate different scenarios. These simulations are in agreement with the laws mentioned, and they shed light on the fundamental truths that are the CAUSES of the numerous resulting EFFECTS that we observe.
A couple of notes regarding my simulations. They assume a constant temperature for the entire system. I also plugged in an arbitrary rate of permeability across the membrane, which only affects the time period in actual time and simulation iterations required to achieve the outcome, but has no impact on the final results in terms of moles, absolute and partial pressures, concentrations, equilibrium, etc. I simplified ambient air to 79% nitrogen, 21% oxygen and 0% CO2. I also factored in differing rates of permeability using Graham's law, so that O2 permeates 1.17X faster than CO2 and Nitrogen permeates 1.25X faster than CO2 (according to their molecular weight, i.e. density). I assumed uniform dispersion/mixing of gases relative to the time required for diffusion.
Here are the summary results of these laws and simulations, as well as corollaries derived from the facts, and clarification for some of the popular myths being propagated. I welcome any counter-proof to anything I have stated.
The cited laws (not me) establish that the kinetic energy of every gas molecule is proportional to its temperature. For a given temperature... Lighter gas molecules have the same KE as heavier gas molecules. KE=1/2mv^2, therefore, heavier gas molecules move slower than lighter molecules. Pressure does not affect the KE of a particular gas molecule. Pressure does affect the rate at which gas molecules will strike a barrier, since there are more molecules per unit volume at higher pressure.
The motion of gas molecules is random motion due to kinetic energy. Diffusion through a permeable membrane results from this random motion, and the rate of diffusion through it is dependent on the probability of a gas molecule colliding with the membrane. So, more moles per unit volume will result in more diffusion through a membrane. This fundamental truth explains everything else.
Please read this point to the end: The motion of gas molecules, as well as the probability of collision with the membrane is unaffected by anything on the other side of that membrane. Meaning, the rate at which gas molecules diffuse FROM LEFT TO RIGHT through a membrane has NOTHING to do with the concentration/partial pressure of that gas on the other side. It is totally dependent on the number of moles of that gas on the left side of membrane. Same goes for the right side. The EFFECT of that fact is that a higher concentration of a particular gas on the left side will result in more molecules of that gas diffusing from left to right than in from right to left. Eventually the concentration will build up on the right side until equilibrium is reached, and the diffusion of molecules is the same in both directions. But this equilibrium doesn't cause this diffusion to cease in either direction, it merely becomes the same number in both directions. There will always be molecules of that gas moving from left to right and right to left at a rate dependent only on the number of molecules on the respective side.
There is no motivating force to drive oxygen molecules from one side to the other because of any concentration imbalance. It is simply kinetic energy and random probability that moves the molecules. If there is 21% oxygen present on the left side, the number of moles of O2 per unit time diffusing FROM LEFT TO RIGHT will be the same number whether there is zero O2 on the other side, or 99% O2 on the other side. Same goes for the right side, so the EFFECT will be a NET DIFFERENCE in diffusion that is solely dependent on the difference between the number of moles of O2 on the left vs the right. So for the case of ambient air on the left of the membrane (where O2 remains at 21%), the number of moles of O2 per unit time diffusing LEFT-TO-RIGHT will be constant regardless of the O2 concentration/partial pressure/build-up on the other side. As O2 builds up on the right side, the number of moles diffusing right-to-left increases and only the NET difference in molecules moving through the membrane will diminish.
Eventually there will be the same number of moles of O2 on both sides of the membrane, and things remain at equilibrium since the same number of molecules will be moving from the left side to the right as in the opposite direction. (The net will be zero but the number of molecules crossing the membrane in either direction will still be a function only of the total number of moles on either side).
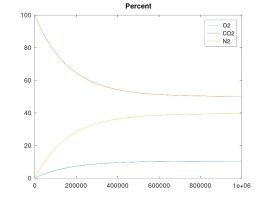
Further, while the NUMBER OF MOLES OF O2 will eventually equalize to the same number on both sides, this does not require that the concentration must be equal on both sides. O2 concentration will be equal on both sides only if the absolute pressure is the same on both sides. But if the the absolute pressure on the right side happens to be higher than the left, that equilibrium point of the same number of moles of O2 on both sides will actually result in a lower concentration of oxygen on the right side. This example case can exist if the right side pressure is maintained with a regulator and a continuous source of CO2. I am providing a simulation for this case that demonstrates a lower concentration of O2 on the right side of the membrane (due to higher absolute pressure), while the diffusion of O2 molecules is across the membrane is equal in both directions (i.e. equilibrium, since the number of O2 moles is equal on both sides).
My simulations also include the case of a balloon, where the balloon is filled with CO2 to varying pressure levels. These balloon simulations all show the balloon pressure eventually approaching ambient (i.e. deflating). The sims also demonstrate the case where the balloon pressure can actually increase for a time - which was enlightening to me. This case can occur due to differences in the rate of permeability for the different gases. In this simulation, Nitrogen and Oxygen start to enter the balloon faster than CO2 can permeate out, and the pressure increases until the build up of O2 and N2 inside the balloon begin to lower the net diffusion of those gases to the point they equal the rate of CO2 diffusion out. After that point the rate of CO2 diffusion outward dominates and the pressure inside the balloon begins to drop until it reaches ambient, at which point the gas in the "deflated" balloon is the same 79%/21%/0% N2/O2/CO2 mix as in the ambient air. Also, the initial pressure increase into the balloon will NOT occur if the starting CO2 pressure inside the balloon is high enough such that the increased number of CO2 molecules inside the balloon results in more CO2 molecules permeating out than O2 and N2 permeating in (despite the higher rate of permeability for N2 and O2 compared to CO2). This will be the case if the CO2 balloon initial pressure is anything above 1.2332 ATM. At anything above 1.23 ATM, there will always be a NET movement of gas molecules out of the balloon and pressure will always drop (even though O2 and N2 are still permeating into the balloon).
The other simulations model the case where the enclosed volume is maintained at a constant pressure with a continuous supply of CO2 gas - which is the example of a keg fed with a CO2 bottle. These simulations clearly show that, while my rationale for the forces that cause these gas molecules to move and diffuse was accurate, my theory about the final effect was in error. The fact is that O2 will tend to diffuse into the higher pressure CO2 line until the number of O2 molecules per unit volume is equal on both sides. The result is that the O2 concentration will be lower than for ambient air if the regulator pressure is higher than ambient. Again, the number of moles of O2 will equalize on both sides, regardless of regulator pressure. As the regulator pressure is close to ambient that equal number of moles will cause a similar concentration, but as the regulator pressure is increased, that same number of moles results in lower concentration, albeit, still a significant percentage since practical regulator pressure is only 1.5X to 2X that of ambient, so maybe 10.5 to 14% O2 concentration at equilibrium.
------------------------------------
The simulations show the eventual conditions of equilibrium given enough time. The missing piece is relating those simulation curves to an actual timeline in terms of days and hours, since I don't have actual permeability numbers for a PVC hose, o-rings etc. The impurity of the CO2 source is also a real-world factor. And the ultimate question is how much of that O2 is actually get dissolved into the beer over the normal time and drain rate of a typical keg (my friends have managed to help empty my last 4 kegs with 1 week of putting them on CO2. I blame the NFL.)
Which is why I intend to do an experiment: fill two kegs 1/2 way with enough sugar water and yeast to yield a relatively oxygen free starting point. Keep both kegs connected to PVC CO2 lines - one without supplying any new CO2 to the line, the other supplying 15 psi CO2 over ambient. Allow both kegs to sit like this for maybe 2 months. Take samples from each (maybe 4 to 8 tests over the 2 month period) and use a Winkler/LaMotte dissolved oxygen test kit to measure dissolved oxygen. This should reveal how far along the theoretical O2 intrusion curve things progress over a typical time period and keg volume. It would probably be beneficial to have a 3rd keg and do a shake under pressure to force carbonate quickly and do a D.O test to establish the purity of the CO2 source to begin with (possibly that can be deduced form the other two test cases?)
Any advice on whether I should buy a test kit with a resolution of ppb or ppm???