beervoid
Hophead & Pellet Rubber
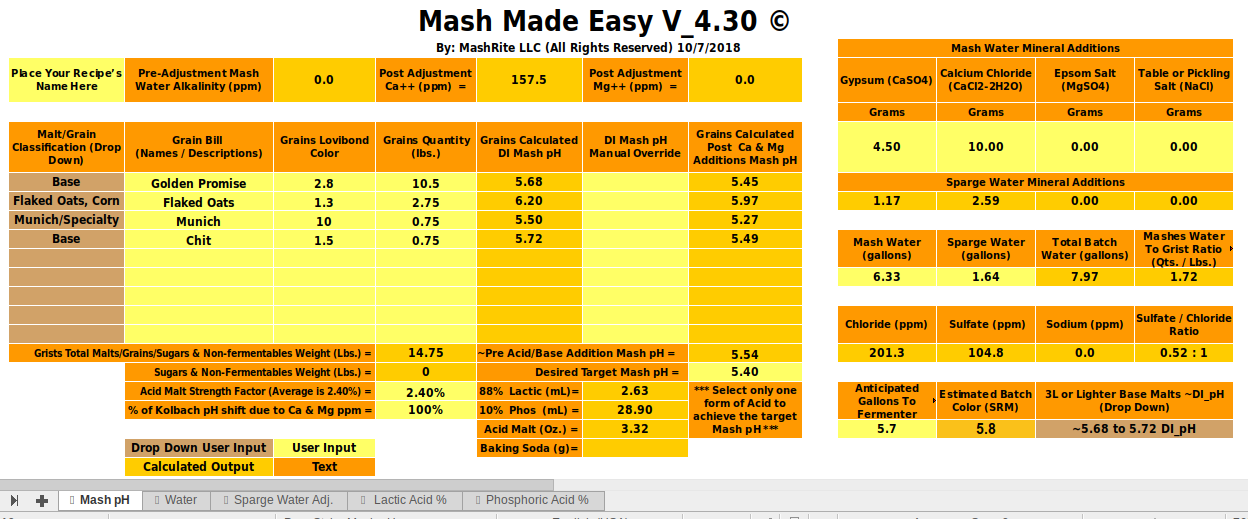
Hello everyone I've been trying to wrap my head around BruNwater as I noticed that my PH calculations are off as compared to the Mash Made Easy spreadsheet.
Also checked with brewersfriend.
Anyone here that could shed some light on this topic as to why BruNwater which I always believed to be the most accurate doesn't calculate the same PH?
Made Easy sheet which calculated that I should have a PH of 5.53
Brewersfriend gave me a PH of 5.45
While BruNwater gives me a PH of 5.27
I'm not sure what im doing wrong here. My water is RO and I add calcium chloride and sulfate additions to get about 150ppm calcium, 100ppm sulfate and 200ppm chloride.
I've currently input the following data in both brunwater and mash made easy.
Mash water: 6.33 gallon
Sparge water: 1.64 gallon
Chloride: 7.5grams (for BruNwater)
Chloride: 10 grams (for mash made easy)
Sulfate: 4.5 grams for both
Grain Bill:
10.5 lbs Golden Promise 5.9EBC
2.75 lbs Flaked Oats 2EBC
0.75 lbs Munich 25EBC
0.75 lbs Chit Malt 2.5EBC
Also checked with brewersfriend.
Anyone here that could shed some light on this topic as to why BruNwater which I always believed to be the most accurate doesn't calculate the same PH?
Made Easy sheet which calculated that I should have a PH of 5.53
Brewersfriend gave me a PH of 5.45
While BruNwater gives me a PH of 5.27
I'm not sure what im doing wrong here. My water is RO and I add calcium chloride and sulfate additions to get about 150ppm calcium, 100ppm sulfate and 200ppm chloride.
I've currently input the following data in both brunwater and mash made easy.
Mash water: 6.33 gallon
Sparge water: 1.64 gallon
Chloride: 7.5grams (for BruNwater)
Chloride: 10 grams (for mash made easy)
Sulfate: 4.5 grams for both
Grain Bill:
10.5 lbs Golden Promise 5.9EBC
2.75 lbs Flaked Oats 2EBC
0.75 lbs Munich 25EBC
0.75 lbs Chit Malt 2.5EBC
Last edited: