LemonJelly
Active Member
I've been doing a lot of research and reading about water reports and changing your water profiles with programs like BeerSmith and Bru'n Water. Everytime I try inputting my numbers, they seem way way off of what other profiles are. Is my water really that far off, should my sodium be that high? or I'm reading the report wrong? can anyone help me write a profile good for hoppy beers with my water?
Also I consider my water very good tasting, coming from someone who is pretty picky about drinking water taste.
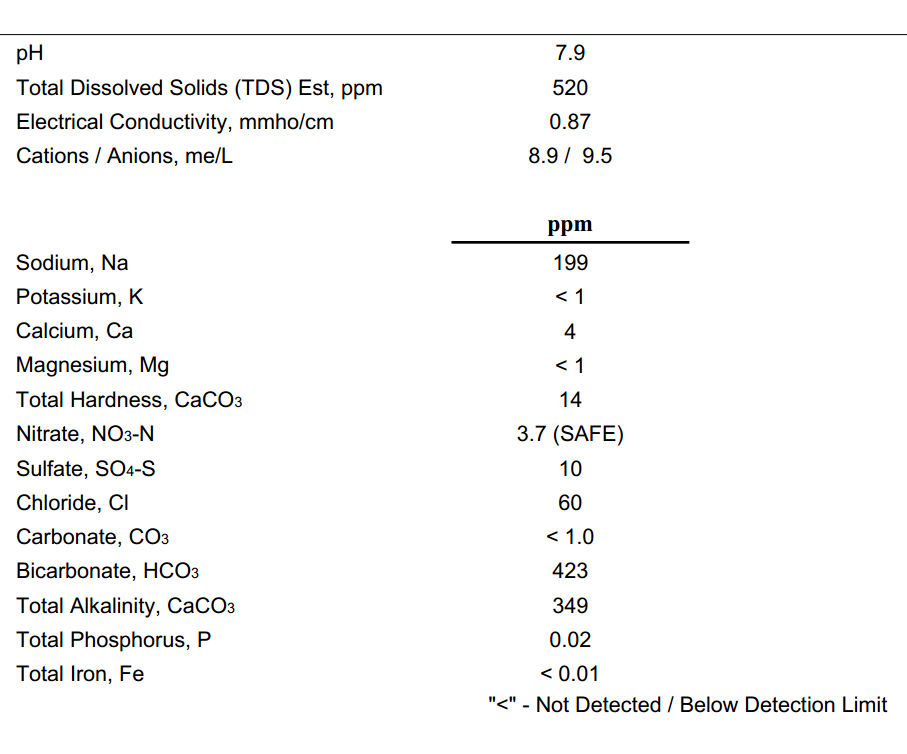
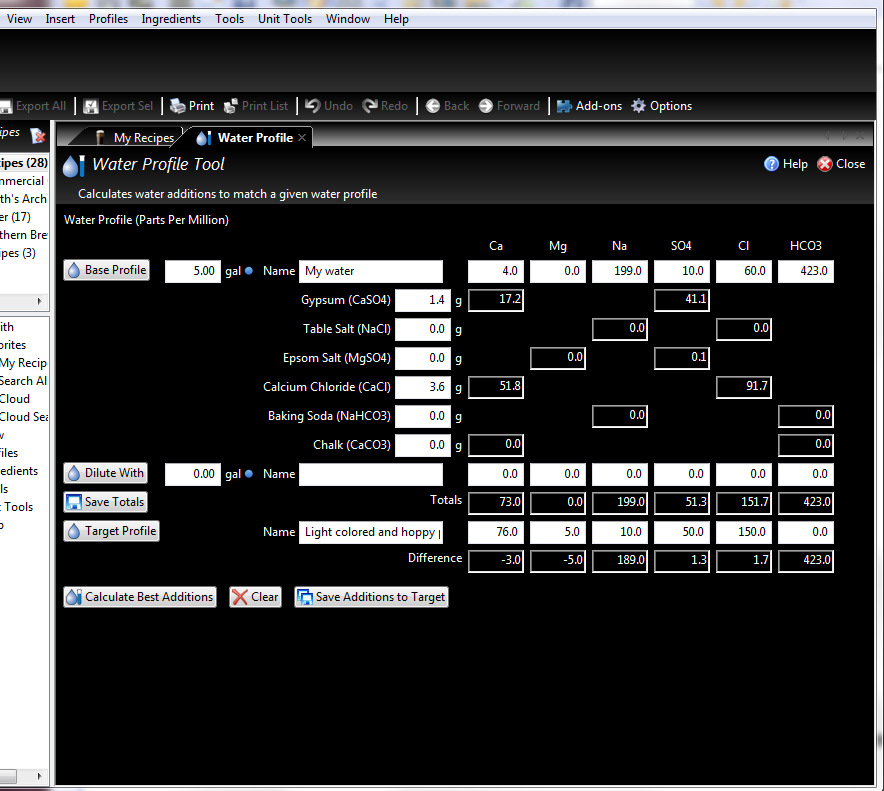

Also I consider my water very good tasting, coming from someone who is pretty picky about drinking water taste.





