This thread is 112% too mathy for me.
It's going to get worse in a big hurry.

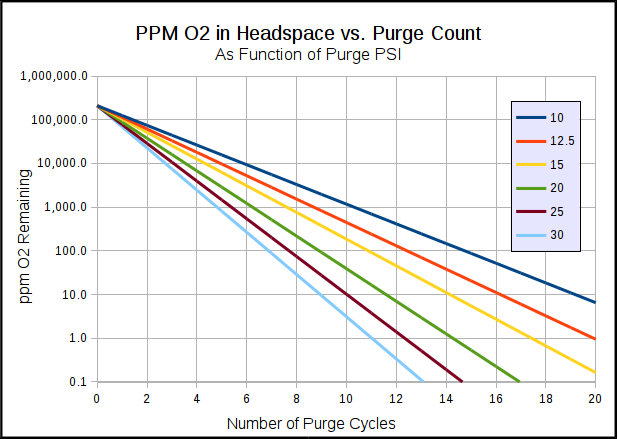
My previous purge dilution charts and tables are based on % or ppm of O2 in the headspace. These are easy to understand since they are independent of headspace volume or beer volume. but, what really matters for beer stability (storage life) is Total Packaged Oxigen (TPO), and to determine TPO you need to know the mass of beer, the mass of O2 in the original beer, and the mass of O2 in the headspace (which depends on the headspace volume and O2 concentration in the headspace.) So, ppm O2 in the headspace is only part of the answer.
TPO is equal to the ratio of mass of the total O2 in the keg to the mass of the beer in the keg. Commercial brewers measure TPO in ppb (parts per billion.) Dissolved Oxygen (DO) is equal to the ratio of the mass of O2 in the beer to the mass of the beer. The TPO is equal to the DO (measured in ppb) plus the ratio of O2 mass in the headspace to beer mass (also in ppb.)
To prevent premature degradation of beer flavor due to packaged O2, the Total Packaged Oxygen (TPO) should be less than about 150 ppb (0.15 ppm) (ref: page 21 of
http://www.craftbrewersconference.com/wp-content/uploads/2015_presentations/F1540_Darron_Welch.pdf.)
Let's do a little math. If we assume the beer starts with 100 ppb of DO, then the headspace contribution to TPO should be less than 50 ppb, based on the mass of the beer, not the volume of headspace. If we have a beer with an FG of 1.009, then the total O2 allowed is:
1.009 kg/L * 150e-9 = 1.5135e-07 kg/L = 1.5135e-04 g/L = 0.15135 mg/L
2.5 gal of beer is 9.46 L, so the total allowed O2 in the keg would be:
1.5135e-04 g/L * 9.46 L = 14.323e-04 g = 1.4323 mg
And the total allowed O2 in the headspace would be:
1.4323 mg * 50 ppb / 150 ppb = 0.477 mg
Air at 0˚C (32˚F) has a density of 1.293 g/L. A "five" gal keg has a total volume of about 5.3 gal, so with 2.5 gal of beer, the headspace volume is about 2.8 gal, or 10.6 L. Air is 21% by weight O2. So, if we get 20% air in the headspace after removing the lid, making an addition, and replacing the lid, the mass of O2 in the headspace will be:
1.293 g/L * 10.6 L * 0.21 * 0.20 = 0.576 g => 576 mg
This is way more than the 0.477 mg target for O2 in the headspace. Thus we must dilute the O2 in the headspace by:
576 mg / 0.477 mg = 1208 times reduction in O2
The dilution equation for pressurized to atmospheric purging is:
Dilution = ((P[atm] + P[purge])/ P[atm]) ^ N, where N is the number of purge cycles
We can solve for the required N by doing some algebra on the above equation to get:
N = ln(Dilution) / ln((P[atm] + P[purge])/ P[atm])
If we purge at 30 psi, then for the dilution target of 1208, the equation becomes:
N = ln (1208) / ln((14.7 psi + 30 psi) / 14.7 psi) = ln(1208) / ln(3.041) = 6.38
We can't do a fractional number of purges, so we need to round N up to 7. Thus, you need seven purges at 30 psi to get the headspace TPO contribution down to less than 50 ppb for the assumptions used.
Brew on