Hey all. What are your procedures for plate streaking? Are you using a pure yeast colony on the loup to streak or are you diluting a pure colony in sterile water first? I'm just getting into streaking to get a pure strain and rid bacteria. I tested using a pure colony on the loup yesterday, I have been diluting first but I was getting varied results.
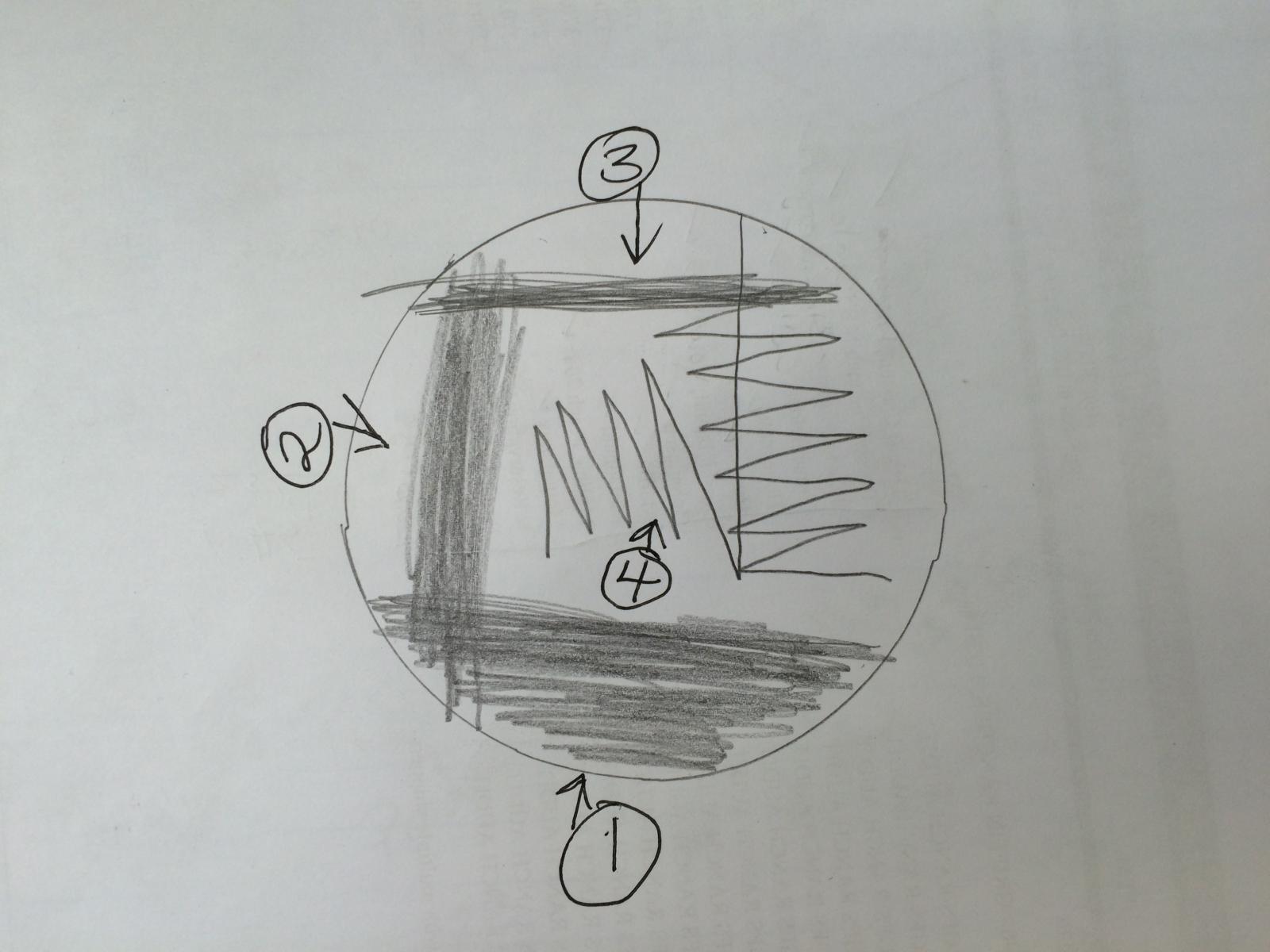
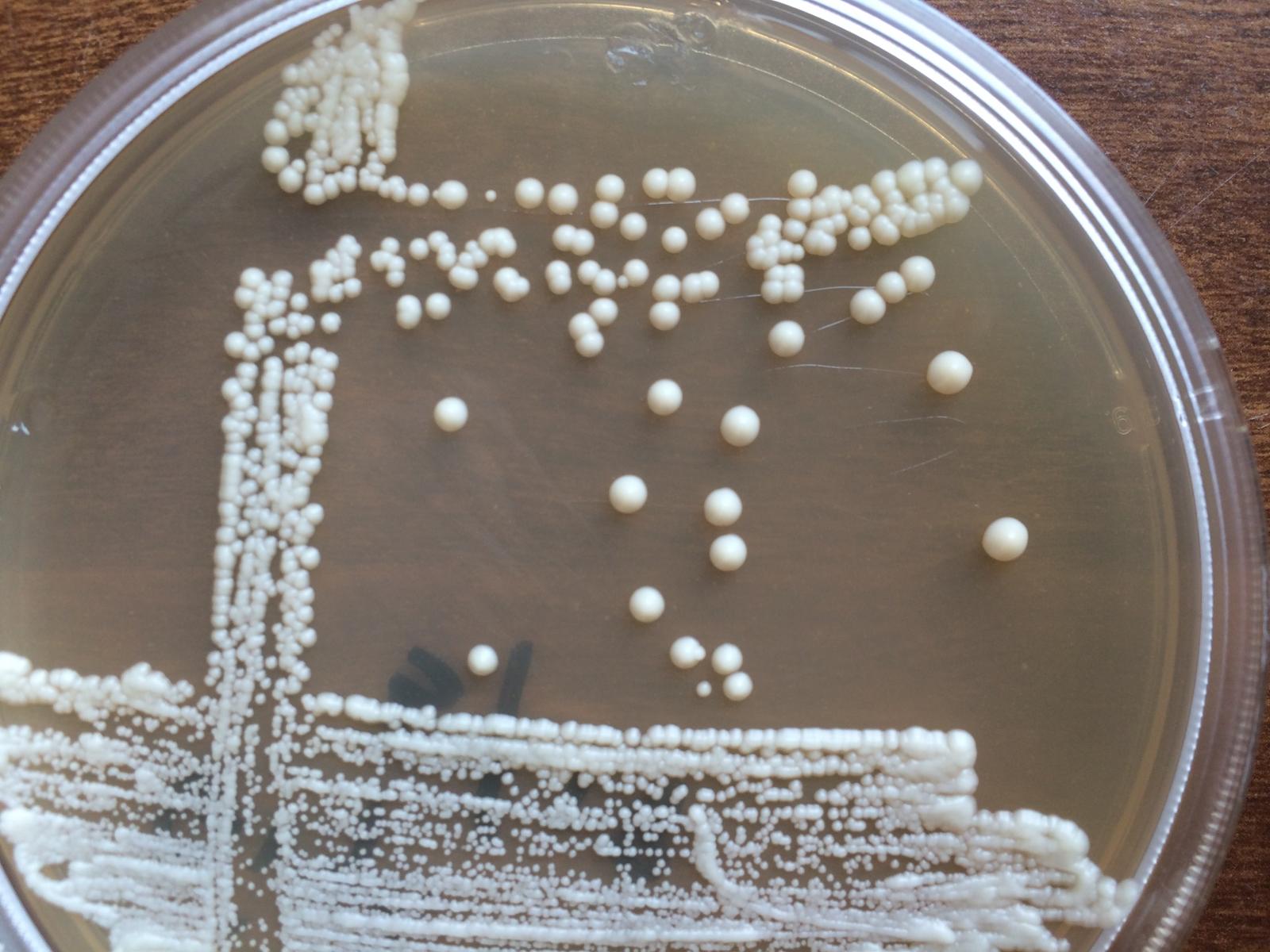
Nice picture. So the idea behind plate streaking (aka dilution streaking) is to isolate pure colonies. So if you have an isolate from a slant, a culture from bottle dregs, captured wild yeast, or want to isolate from a nice fermentation, etc., you can use this technique to get individual colonies that have formed from a single cell. The idea is to spread the organisms so thin that these individual colonies form. Below is an illustration of the way I do it (there are other methods out there) and below that is an image of the results.
First get a very small amount of yeast (let's say a slant) on your loop. If you can see it, it's enough. Then spread onto the first section of the plate by moving the loop back and forth without picking it up until the section is spread thin. Then flame the loop and spread from one end of the first section into the second section in the same manner, flame and move to the third section overlapping the second section. After you have spread the third section and without picking up the loop continue in a zigzag pattern as illustrated. Flame the loop and in a single swipe move through section 3 including the zigzag and again without picking up the loop continue in a zigzag pattern into the final (#4) clean area of the plate. The single colonies will most likely form in the zigzag pattern of section 3, however if things are too thick your single colonies may form in section 4.
If you were isolating from a culture (dregs, wild ferment, etc) more volume is needed, so use 3 to 6 loopfulls of the culture for the first step. Hope this helps.