I just tasted a batch while transferring to a keg and it had an awful metallic and fusel taste. Never experienced these flavors before so not quite sure what happened, there were some unique things about this batch which I've listed bellow. The beer was a really basic American Pale Ale with White Labs WLP001. According to the BJCP Fault List the characteristics and causes are:
Metallic - Check water for metallic ions. Reduce water salts. Check equipment condition for rust. Make sure stainless steel equipment is properly passivated. Fully rinse sanitizer. Try using RO water and add salts as needed.
Fusel - Lower fermentation temperature. Pitch a sufficient quantity of healthy, active yeast. Check for infection. Try a different yeast strain.
There are three main suspects at this time:
1) Infection - Blowoff tube got pinched and the stopper was blown out. Took about a day for me to notice. The only time I've ever had an infection this is what happened, however it was an extremely sour/acidic taste and not a metallic/fusel. Thinking this is the most likely issue.
2) First time using copper HERMS coil - could this impact some off flavors? It was my immersion cooler before and never had any issues, but this was the first time wort was pumped thought it. I did not soak the inside with the white vinegar mix that I used on the outside.
3) First time building water from completely RO - I used the EZ_water_calculator spreadsheet that many people on here use and this is what I came up with for the american pale ale. At the time it seemed like quite a bit of minerals but I don't have much experience with salts to go by.
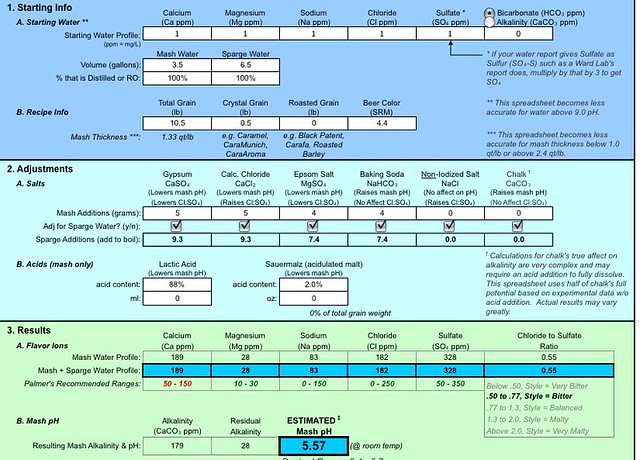
Thoughts on what was mostly likely the culprit? I'd hate to have another couple of bad batches due to my lack of understanding of water salts. Thanks guys.
Metallic - Check water for metallic ions. Reduce water salts. Check equipment condition for rust. Make sure stainless steel equipment is properly passivated. Fully rinse sanitizer. Try using RO water and add salts as needed.
Fusel - Lower fermentation temperature. Pitch a sufficient quantity of healthy, active yeast. Check for infection. Try a different yeast strain.
There are three main suspects at this time:
1) Infection - Blowoff tube got pinched and the stopper was blown out. Took about a day for me to notice. The only time I've ever had an infection this is what happened, however it was an extremely sour/acidic taste and not a metallic/fusel. Thinking this is the most likely issue.
2) First time using copper HERMS coil - could this impact some off flavors? It was my immersion cooler before and never had any issues, but this was the first time wort was pumped thought it. I did not soak the inside with the white vinegar mix that I used on the outside.
3) First time building water from completely RO - I used the EZ_water_calculator spreadsheet that many people on here use and this is what I came up with for the american pale ale. At the time it seemed like quite a bit of minerals but I don't have much experience with salts to go by.

Thoughts on what was mostly likely the culprit? I'd hate to have another couple of bad batches due to my lack of understanding of water salts. Thanks guys.

