briggssteel
Well-Known Member
Ok I am new to adjusting my water and would like some advice. First of all here is my report average:
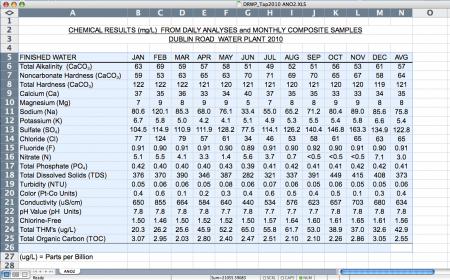
Calcium:35
Magnesium:8
Sodium:75
Sulfates:122
Chloride:65
Total Alkalinity:57
PH:7.8
Total Hardness:121
My recipe is a Robust Porter:
11 pounds Marris Otter
.25 pounds Victory Malt
.75 pounds Crystal 70L
.75 pounds Black Malt
.50 pounds Chocolate
.25 pounds Carapils
Add 1.7gms or 1/2 teaspoon of Calcium Chloride putting my Calcium at 60 and my Chlorides at 108.
Mash at 154 for 1 hour
Aiming for a PH of 5.3 or 5.4
Now, I've plugged all of this into brewing water and have some questions. It says right now my mash PH should be 5.65.
1. It's not asking for my water PH. Does it assume it's around 8?
2. I thought dark grains were supposed to drive down the Mash PH significantly ending up too low. This is too high.
3. If I add 3ml of 88% Lactic Acid it will drop my PH to 5.45, but I have my doubts about adding that much lactic acid and it not affecting the flavor of my beer. Plus it drops my RA to -122. I thought RA was supposed to be high for a porter?
I guess my question is does the EZ Water calculation seem right? This is my first time adjusting water and I don't want to go nuts. Just get my Calcium to correct levels and have my mash PH be in the correct range. Thanks in advance.
Oh, I also had a side question. I am moving my brewing to another location. The water is run through a water softener. Should I use this water? Wouldn't a water report that I get be way off after it's been run through the softener? Thanks.
Calcium:35
Magnesium:8
Sodium:75
Sulfates:122
Chloride:65
Total Alkalinity:57
PH:7.8
Total Hardness:121
My recipe is a Robust Porter:
11 pounds Marris Otter
.25 pounds Victory Malt
.75 pounds Crystal 70L
.75 pounds Black Malt
.50 pounds Chocolate
.25 pounds Carapils
Add 1.7gms or 1/2 teaspoon of Calcium Chloride putting my Calcium at 60 and my Chlorides at 108.
Mash at 154 for 1 hour
Aiming for a PH of 5.3 or 5.4
Now, I've plugged all of this into brewing water and have some questions. It says right now my mash PH should be 5.65.
1. It's not asking for my water PH. Does it assume it's around 8?
2. I thought dark grains were supposed to drive down the Mash PH significantly ending up too low. This is too high.
3. If I add 3ml of 88% Lactic Acid it will drop my PH to 5.45, but I have my doubts about adding that much lactic acid and it not affecting the flavor of my beer. Plus it drops my RA to -122. I thought RA was supposed to be high for a porter?
I guess my question is does the EZ Water calculation seem right? This is my first time adjusting water and I don't want to go nuts. Just get my Calcium to correct levels and have my mash PH be in the correct range. Thanks in advance.
Oh, I also had a side question. I am moving my brewing to another location. The water is run through a water softener. Should I use this water? Wouldn't a water report that I get be way off after it's been run through the softener? Thanks.