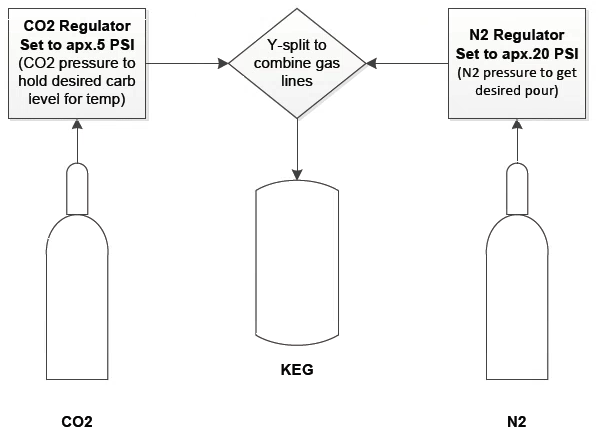
Title says it all.
I have a 20 lb nitro tank that I picked up from one of the welding supply shops in town. It's beergas mix, but I have no idea what the percentages of Nitro to CO2 are. I've never had it refilled, but it's about out. I get my CO2 refills from a competing weld supply shop, and they'll exchange the tank I have. They'll also do whatever percentage mix I want.
If I knew what was in the tank I've been using, I may just replicate that. Nonetheless, it seems like a good opportunity for me to dial in my nitro setup, I just don't know what an ideal mix is for a kegorator situation. I'm pretty sure beergas mixes usually range between 70-80% N2 & 20-30% CO2.
Any advice on the butter zone would be greatly appreciated.
I have a 20 lb nitro tank that I picked up from one of the welding supply shops in town. It's beergas mix, but I have no idea what the percentages of Nitro to CO2 are. I've never had it refilled, but it's about out. I get my CO2 refills from a competing weld supply shop, and they'll exchange the tank I have. They'll also do whatever percentage mix I want.
If I knew what was in the tank I've been using, I may just replicate that. Nonetheless, it seems like a good opportunity for me to dial in my nitro setup, I just don't know what an ideal mix is for a kegorator situation. I'm pretty sure beergas mixes usually range between 70-80% N2 & 20-30% CO2.
Any advice on the butter zone would be greatly appreciated.















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)