SMD
Well-Known Member
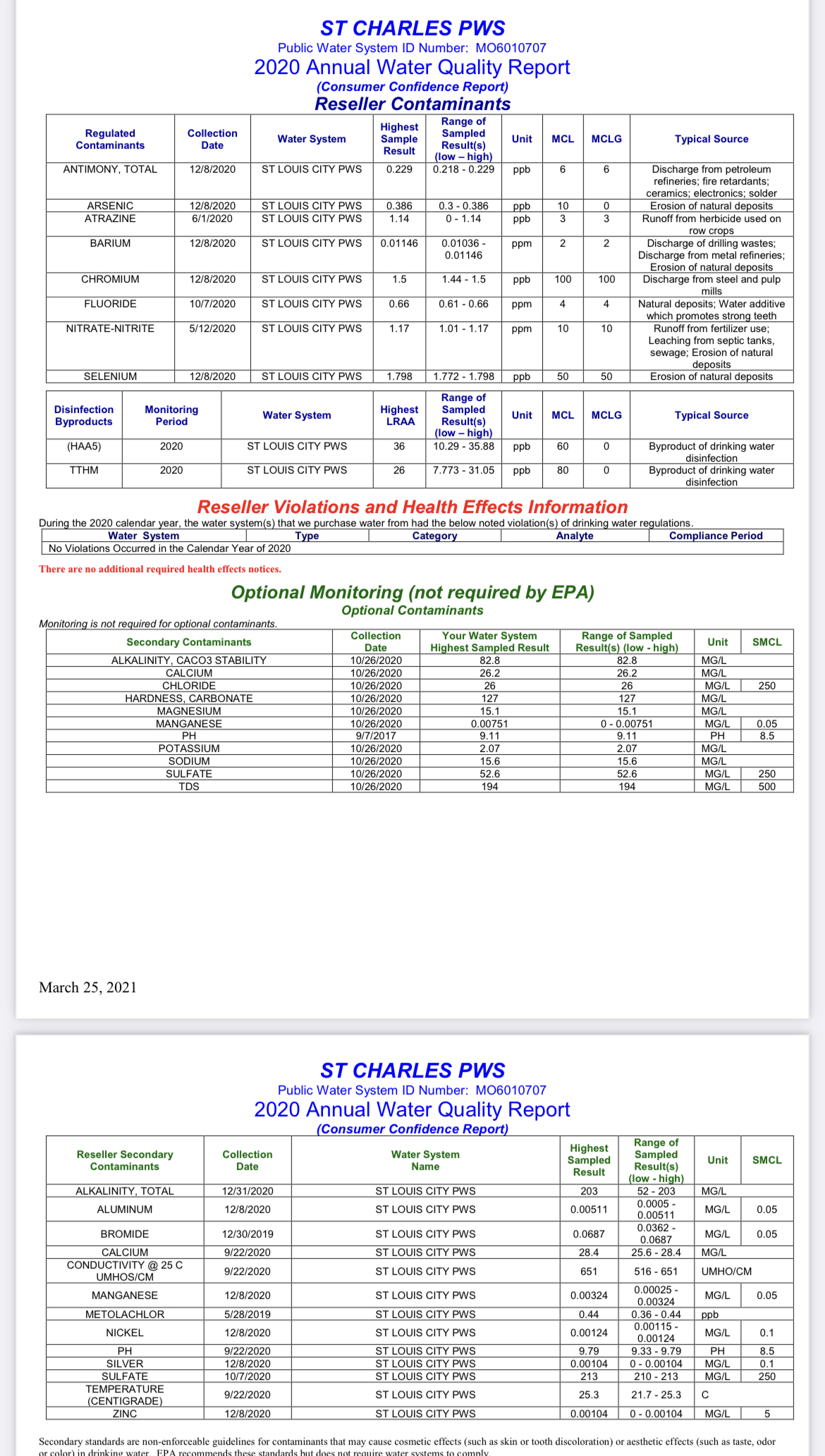
Can anyone advise which figures I should be using to calculate water chemistry from my water report. There are two separate sections that list Calcium, Sulfate and Alkalinity levels. I would like to make calculations based on the correct source minerals. Thanks in advance!