Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
5.51
Thanks A.J.!

5.51
It now seems all these recent posts regarding ph are more directed at the fact people are taking readings at 10-20 mins and it's matching what there software is suggesting. So is you argument that the ph needs to be taken at 30 mins if using newer software and the old method is incorrect? If so we have all wasted alot of bandwidth debating seemingly the same thing over and over and I think rewording the question would have prevent the ongoing debate. That being said these threads in the last few days are the first time I've heard of doing it that late. Ive never noticed a significant change in the ph from 10-30 mins but I've also only been concerned about staying in range. I also use a herms system and run it at full speed recirculation so it might help with how much time it takes to get a accurate reading as the liquid is turned over much faster than just letting it sit. Cheers
I was asking a question. You seem to think everyone is challenging you and never actually answer the questions. Take a breath. You and the others have forgotten more about this topic then I'll ever know. Jeez what's so hard for you to understand. I'm asking a simple question because I genuinely stiil don't understand what this huge debate is about. I ask a simple question and get a huge long scientific answer that most including myself don't understand. Can you please answer my question directly and simply. All I've ever said over and over and over again is that the software I was using appeared to be working fine. All my question to you is what software do you recommend I use and what time do you suggest I check my pH. It's that simple dude come on wake up. It's not a challenge.If you are happy to accept a false low early pH reading taken when saccharification is incomplete and reactions are still actively happening, merely because it works for your preferred software (and obviously for your preferred general to specific chosen water to grist ratio, which is a demonstrable tripping point for your softwares output precision) rather than achieve a more correct mash pH, then by all means remain happy and continue as you have been doing. But this thread is seriously attempting to establish the strict criteria by which valid mash pH measurements can be achieved (plus determine if they even can) across multiple people making multiple batches, and the last thing that statistical significance (the entire point of this thread) needs by which to damage it and make it impossible to achieve is to have everyone who contributes on a different page rather than everyone on the same page.
But despite your happiness, if your software says something on the order of 2.4 mL of lactic acid is correct to hit a 5.4 mash pH target, and A.J. deLanges proton balancing software says something on the order of 5.9 mL is required (quite similar as to what BS3 predicts from your testimony in other threads by the way), and if A.J.'s software is using hard science based upon measured chemical reaction reality (and D.M. Riffe, is doing likewise, wherein BS3 is using D.M. Riffe), then shouldn't you be willing to take a step back and reflect upon the potential implications of this a bit, rather than ever boldly charging forward and stating your position that sampling at 10 minutes always works for you and your preferred software so therefore it not only should, but must, be made to work the exact same way for everyone else as well, with the clear implication that their software and science efforts and mash pH sampling times are flawed?
I was asking a question. ... snip ... All my question to you is what software do you recommend I use and what time do you suggest I check my pH. It's that simple dude come on wake up. It's not a challenge.
Thanks for the easy to understand response. Do you think it's possible the reason people are having issues with bs3 is because there taking there Reading's at the 10min mark? I only ask because I really really want a all in one solution and didn't have luck with bs3? Cheers1) MpH or Brewer's Friend in grist mode, followed by gen 2 when it actually appears. Brewer's Friend accepts DI_pH values.
2) 30 minutes or later during the mash.
I've responded to this post before but am doing so again on a different part of it i.e. that quoted above. I was looking at the Riffe formula the other day and got a "hey, that looks familiar!" twinge. And indeed it is. This is going to get a bit technical so don't feel bad if you are not one of the small group interested in this stuff (e.g. people developing spreadsheets - maybe this should be under Brewing Software.1) BS3 is gen 1, but as stated above, if it is using Riffe's highly respected formulas (as RPIScotty attests), it has an undeniably high pedigree in that regard.
















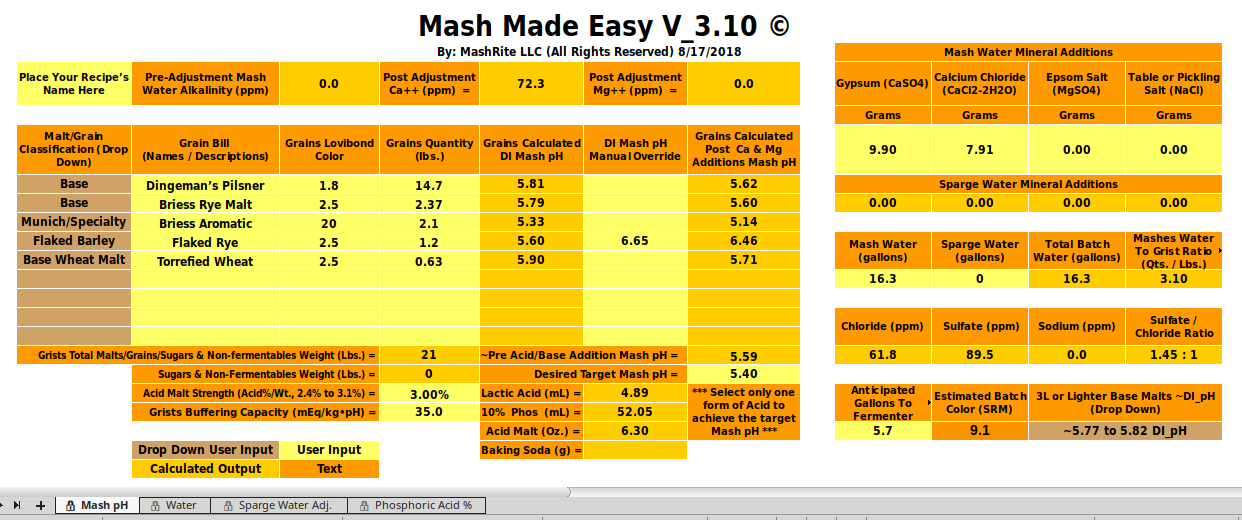
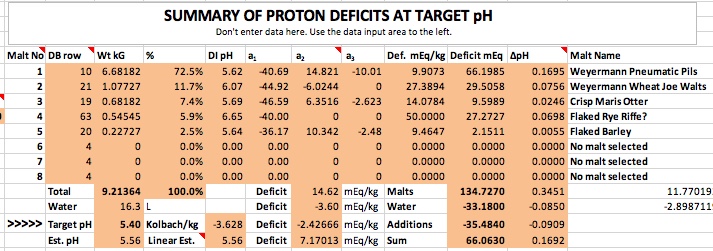
@Silver_Is_Money , thank you for looking at this. I definitely had items wrong. I had the grist buffering capacity at 34.0. My anticipated gallons to fermenter was 10.5 gallons.
I had:
- Dingeman's Pilsner 1.6 Lovibond Color
- Briess Rye Malt 3.7
- Briess Aromatic Malt 20
- Flaked Rye 3.0
- Torrefied Wheat 1.50
It would have been interesting to see if a sample drawn at fully 60 minutes into the mash would have come in at about 5.61 to 5.63 pH.
I will take a 60 minute measurement in the future. Thanks for the suggestion.
@CodeSection for a 10 gallon batch using the grains you mention. I get a predicted mash pH of 5.50 without adding any lactic acid when just adding 9.9g gypsum and 7.1g of calcium chloride. When adding 3.1ml lactic acid it changes to 5.41 pH. How sure are you of your actual pH measurements?I definitely had items wrong. I had the grist buffering capacity at 34.0. My anticipated gallons to fermenter was 10.5 gallons.
I had:
- Dingeman's Pilsner 1.6 Lovibond Color
- Briess Rye Malt 3.7
- Briess Aromatic Malt 20
- Flaked Rye 3.0
- Torrefied Wheat 1.50
Thank you! Are you interested in taking a DI_pH for your Briess Rye Malt? Mash 50 grams of the malt in 100 mL of distilled (or good quality RO) water at about 146-158 degrees F. for 60 minutes, and take the DI pH reading at room temperature.
@CodeSection for a 10 gallon batch using the grains you mention. I get a predicted mash pH of 5.50 without adding any lactic acid when just adding 9.9g gypsum and 7.1g of calcium chloride. When adding 3.1ml lactic acid it changes to 5.41 pH. How sure are you of your actual pH measurements?
@CodeSection for a 10 gallon batch using the grains you mention. I get a predicted mash pH of 5.50 without adding any lactic acid when just adding 9.9g gypsum and 7.1g of calcium chloride. When adding 3.1ml lactic acid it changes to 5.41 pH. How sure are you of your actual pH measurements?
Please take a reading at 25 - 30 minutes. Nothing wrong with taking another at 60 too but what we need is the 30 minute reading.Thank you! Are you interested in taking a DI_pH for your Briess Rye Malt? Mash 50 grams of the malt in 100 mL of distilled (or good quality RO) water at about 146-158 degrees F. for 60 minutes, and take the DI pH reading at room temperature.
I’m not looking at DI pH in ezRecipe using Riffe’s latest formula. ezRecipe 1.21 is using Gen1 color based predictions based on Riffe’s prior work.Are you considering that the Flaked Rye has a DI_pH of a whopping 6.65 per D.M. Riffe?
Please take a reading at 25 - 30 minutes. Nothing wrong with taking another at 60 too but what we need is the 30 minute reading.
And if you are really interested in helping out repeat the process with 200 mg of sodium bicarbonate added. This will give us the buffering (equally as important as the mash pH) using the Riffe method but with sdium bicarbonate as the 'fiducial malt'.
@CodeSection for a 10 gallon batch using the grains you mention. I get a predicted mash pH of 5.50 without adding any lactic acid when just adding 9.9g gypsum and 7.1g of calcium chloride. When adding 3.1ml lactic acid it changes to 5.41 pH. How sure are you of your actual pH measurements?
Close except for one little detail.
Mash at 50 °C for 60 minutes with frequent stirring. At 30 minutes withdraw a little of the liquid and cool to room temperature as quickly as possible. Measure the pH of that. It might make things a little easier if you used 40 g with 100 mL (that's what I did for the MBAA paper which represents the closest thing to a standard procedure that we have at this time).
Continue mashing for another 30 minutes at 50 °C (total 60 minutes). Cool a sample and measure.
I’m not looking at DI pH in ezRecipe using Riffe’s latest formula. ezRecipe 1.21 is using Gen1 color based predictions based on Riffe’s prior work.
@30 minutes: cooled to 24.6c (76.28F) 5.56 pH
I just don’t get how a measurement of pH 5.56 is possible given the grains and mineral additions plus 3.1ml of lactic on top of that.