Good catch. Wonder if it could at least handle the 15 PSI supported by the Flex+?
That'd be interesting to find out. Which would go first?.....The compression fitting or the gasket?

Good catch. Wonder if it could at least handle the 15 PSI supported by the Flex+?
Well congratulations! I'm many years from that, so I hope you enjoy it
So you're saying to your knowledge, nobody with a Spike CF is able to get chilling down below ~35 with glycol? I just did a forum search and couldn't find anything specific.
I easily crash to 28F in my 10 gallon batches with my CF10 and IceMaster 100 glycol chiller.....
I'd trade you the years away for the retiring.
Yeah--and you'd better believe if I found someone who had, I'd be all over them for details.
Not saying it can't be done, I'm hoping it can. I just haven't found anyone doing it that's reported on it. At least, not yet.
Now, Codesection claims he can do it above, crashing to 28 degrees. I'll be denigrating him in another post and badgering him for details. But then again, @CodeSection has always appeared to be half a bubble off plumb, so who knows what he's thinking.

I've done better in my garage in the winter when ambient gets down near 40 degrees, but that's almost the same as putting the fermenter in a refrigerator.
I was looking at Menards for some 2" thick foam board and finally ran across some that is 2' wide. That's always been my issue--how do you cut a 4x8 sheet of foam board and get anything like square cuts? This gets me almost all the way there: https://www.menards.com/main/buildi...-2-x-8-r-10/526647/p-1444450488436-c-5779.htm
Meanwhile, I'll go harangue on Codesection.
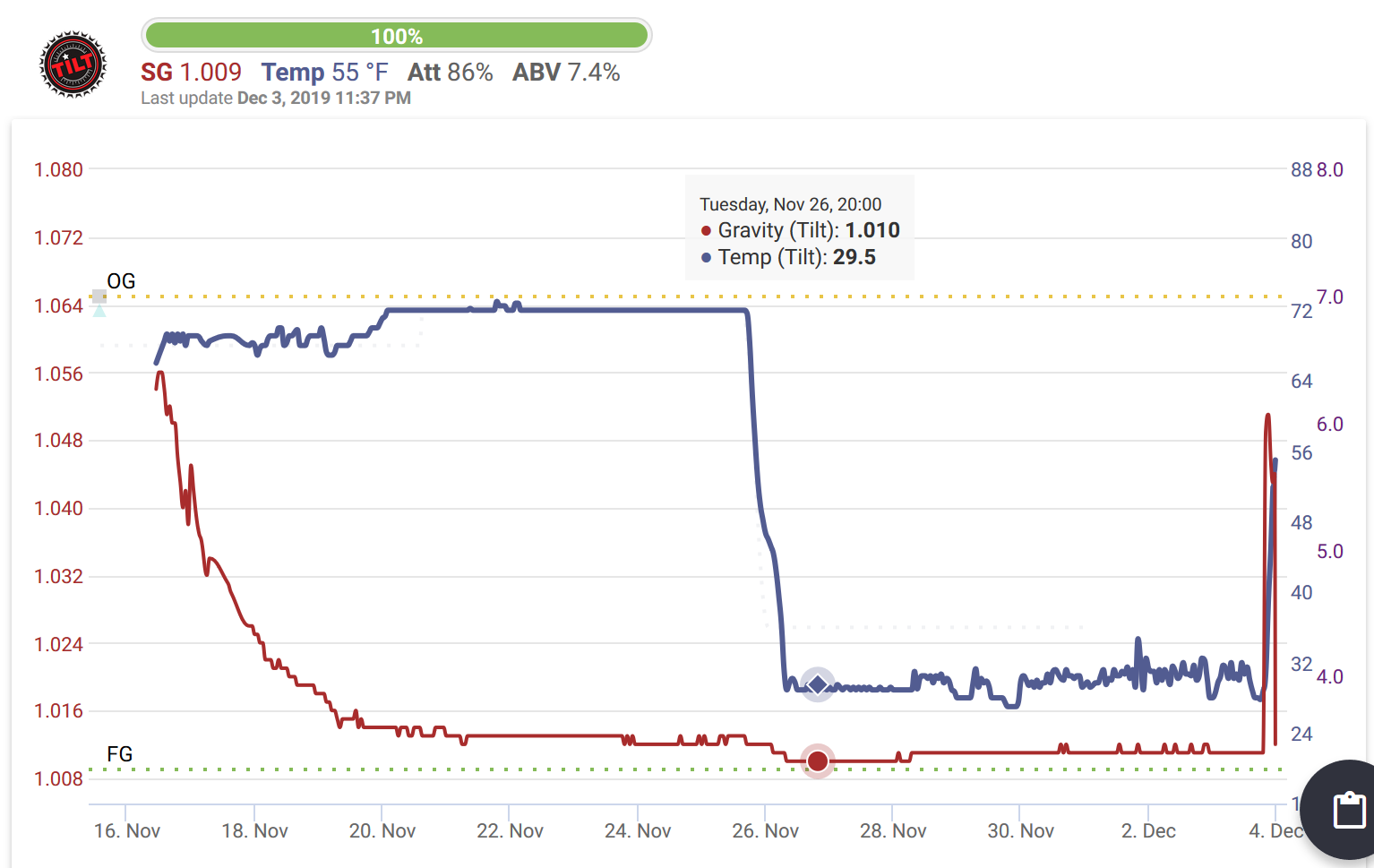

Details, man. What temp is the chiller? Do you get beersicles?
You can't just say something like this without all the pieces and parts!
I've tried so many things. It's my White Whale. Now I'm down to building an insulated chamber for the fermenter. It's no longer important that I chill it to 30 degrees. It's important that I figure out why I can't.
Details, man. What temp is the chiller? Do you get beersicles?
You can't just say something like this without all the pieces and parts!
I've tried so many things. It's my White Whale. Now I'm down to building an insulated chamber for the fermenter. It's no longer important that I chill it to 30 degrees. It's important that I figure out why I can't.

![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)













..... But then again, @CodeSection has always appeared to be half a bubble off plumb, so who knows what he's thinking.

.......
how do you cut a 4x8 sheet of foam board and get anything like square cuts? This gets me almost all the way there: https://www.menards.com/main/buildi...-2-x-8-r-10/526647/p-1444450488436-c-5779.htm
Meanwhile, I'll go harangue on Codesection.
I think you already know that I like to brew higher ABV beer. In the past I have set the chiller between -5c and -8c and to my knowledge, no beersicles yet.
I built my keezer coffin and lid out of oak boards and 1" pink foamboard insulation cut from the 4x8 sheet. I got my cuts pretty darn square by marking it with a chalk line, cutting it carefully with a utility knife, then holding up the edge to the edge of the oak board and shaving it (like you would with a spokeshave) to be exactly flush with the board. I'm pretty anal and I was happy with it.
I built my keezer coffin and lid out of oak boards and 1" pink foamboard insulation cut from the 4x8 sheet. I got my cuts pretty darn square by marking it with a chalk line, cutting it carefully with a utility knife, then holding up the edge to the edge of the oak board and shaving it (like you would with a spokeshave) to be exactly flush with the board. I'm pretty anal and I was happy with it.
Details, man. What temp is the chiller? Do you get beersicles?
You can't just say something like this without all the pieces and parts!
I've tried so many things. It's my White Whale. Now I'm down to building an insulated chamber for the fermenter. It's no longer important that I chill it to 30 degrees. It's important that I figure out why I can't.
Details, man. What temp is the chiller? Do you get beersicles?
You can't just say something like this without all the pieces and parts!
I've tried so many things. It's my White Whale. Now I'm down to building an insulated chamber for the fermenter. It's no longer important that I chill it to 30 degrees. It's important that I figure out why I can't.
These glycol discussions always crack me up. Glass front refri
Maybe you could put the fermenter in an old freezer. They are well insulated. You could run you glycol lines in there like you ran gas lines into your fridge.

I'd trade you the years away for the retiring.
Yeah--and you'd better believe if I found someone who had, I'd be all over them for details.
Not saying it can't be done, I'm hoping it can. I just haven't found anyone doing it that's reported on it. At least, not yet.
Now, Codesection claims he can do it above, crashing to 28 degrees. I'll be denigrating him in another post and badgering him for details. But then again, @CodeSection has always appeared to be half a bubble off plumb, so who knows what he's thinking.

I've done better in my garage in the winter when ambient gets down near 40 degrees, but that's almost the same as putting the fermenter in a refrigerator.
I was looking at Menards for some 2" thick foam board and finally ran across some that is 2' wide. That's always been my issue--how do you cut a 4x8 sheet of foam board and get anything like square cuts? This gets me almost all the way there: https://www.menards.com/main/buildi...-2-x-8-r-10/526647/p-1444450488436-c-5779.htm
Meanwhile, I'll go harangue on Codesection.
@mongoose33 I just had a nice long chat about your issue with a friend of mine who used to teach heat transfer and system balancing on nuclear submarine reactors.
He gave me some stuff to think about. WARNING THERE'S MATH ABOUT HAPPEN!!!
The specific heat formula can be applied here very nicely:
Q=mc∆t - Q is the amount of heat in your glycol system, m is the mass of coolant (determined by the flowrate) c is the specific heat of the glycol (~0.7), and ∆t is temperature of your glycol out of the beer - temperature of your glycol going into the beer.
You can also setup this equation to measure the amount of heat being added by the environment, where your mass is the air, (very low) so call it 1, c is the specific heat of air (~0.2), and your deltaT is the difference between ambient air, and bulk beer temperature. So as long as the Q for your glycol system Q1) is larger than the Q of the environment (Q2) then you can cool your beer. The problem you are having is that the Q2 is growing as the beer cools because the deltaT is growing. So your Q1 is limited by either the flowrate, or the temperature of you glycol (i.e. the ability of your chiller to keep up). Your chiller is huge- it has no problem keeping up, so ignore that. The only thing you can do to boost your Q1 is to boost your flowrate. And it needs to be high enough that your Q1 is greater than your Q2 even when your beer is at 32F and your environment is at 90F.
Your beer SHOULD chill to the temperature of the glycol coming out of your beer - so one of two things is happening that is preventing that for you.
Either the temperature of your glycol coming out of your beer is 38 degrees, which limits how cold your beer can get to 38 degrees. If this is the case, you can lower your glycol temp or increase the flowrate of your pump (buy a bigger pump) and it will solve your issue.
If the temperature of your glycol coming out of your beer is lower than your beer temperature, that means that your beer is gaining temperature from the environment faster than your pump can remove it. The solution is again, to increase the flowrate of your pump (buy a bigger pump)
Incidentally, the notion that ice on your coils insulates them and reduces chiller efficiency is nonsense. That's only true if you are trying to cool the bulk temperature below the freezing point of your beer. Heat energy will be pulled out of your beer to melt that ice (it's no different than dropping ice cubes into your beer). So even if you have a complete beercicle on your coil, your beer will still chill to 32 degrees (28... whatever the freezing point of your beer is)
Also, mixing is a big problem for us, so he suggested a saltwater aquarium mixer being installed in the fermenter. They can be mounted with the motor on the outside of the fermenter and effectively just a fan on the inside (a stir plate more or less). This would help a ton with heat transfer.
Bottom line - I think you just need a bigger pump
I don’t think a bigger pump will help significantly, as the system is conduction limited on the beer side of the coil.
Convection is already sufficiently high for turbulent heat transfer on glycol side of the coil, so instead is limited by surface area for heat pickup. If I remember right, temperature measurements on intake and exit of the coils that @mongoose33 took a while back illustrated a limited temperature increase, which supports this.
The best control volume to draw is around the beer to capture the heat rate in and out. The bit that the cited equations are missing is that they are thermodynamic equations rather than heat transfer equations. They can be manipulated to q=h A deltaT, where h is the convective heat transfer coefficient, A is the surface area, and delta T is the temp gradient. Using the beer as the CV and solving the equilibrium state gives
h_ambient A_ambient (T_amb - T_beer) = h_coil A_coil (T_beer - T_coil).
The beer has some natural convection internally, but assume this to be very low, approaching conduction but with a small supplement due to natural convection.
This triggers a few strategies:
1. Increase circulation in the beer to increase h_coil - sure, but this also increases h_ambient (at least on the cold side of ambient), so not the most effective
2. Change the cold side temp gradient by lowering glycol temperature, this can be done for high ABV beers like @CodeSection does, but is limited at 28 F for 5% ABV
3. Increase the cooling surface area (either through more coils rounds internally or by wrapping fermenter)
4. Decrease the h_ambient via insulation
I’m sure others can look at equation above and develop other ideas based on what they’ve got handy, but my shorthand summary is that the area ratio between cooling coils and conical outside surface needs to be sufficient to balance the h_ambient delta_T, which can itself be lowered by better insulation.
Barring the above suggested time rate of change measurements, you can also use the equation above to at least ballpark how much of a change you need to make based on your present equilibrium state.
I think the biggest bang would be an additional coil run to double the cold side area (similar to inner and outer coil runs in immersion chillers). To illustrate, if your current system gets to 40 F with a 28 F glycol temp (7 C cold delta T) at a 90 F ambient (28 C ambient delta T), then doubling the area (made a swag of area ratio increase from Ac/Aa = 0.2 to 0.4) would allow you to achieve 34 F all other things being equal and taking a bunch of assumptions.
This post is long enough, so won’t list those, but at least wanted to illustrate the concept. If you want to do a calculation on your system, then it would be good to take some measurements of geometries and specific temperatures to reduce the number swags that I used.
Great response thank you
I find it hard to believe that the system is conduction limited given that the specific heat of the glycol is 0.7 and the specific heat of air is 0.2, and the actual difference in surface area between the uninsulated parts sticking out of the neoprene jacket and the cooling coil isn't that great (unless you have a ton of doohickies adding thermal mass to each TC fitting).
Assuming for a moment you are correct, and I'm still noodling on it - could you reduce your glycol temperature much lower, say 20F, and then DECREASE pump speed (throttle the pump) to help increase your rate of heat transfer into the coils with the larger deltaT?
You shouldn't get icing except maybe at the top of the coil with the slower flow rate if I'm thinking about it right.
Edit: You'll get icing as you reach equilibrium and the beer temperature drops because your Delta-t shrinks - still - icecubes cool beer right?
Edit edit : you could also boost the specific heat of your glycol mixture slightly by reducing it from 50/50 to 30/70 (more water)
As to the assumption that neoprenecan be neglected for conical area, it cannot, since its R-value is quite low compared with closed cell foam or other materials used commonly for insulation. It is a speed bump for the heat rather than a blocker.
For reference, I have a jacketed (large cooling surface area) internally insulated (high R value, though I haven’t measured it) conical that keeps beer at 30 with glycol at 28 in a garage that’s 90+...
Ah good point on the neoprene, in that case yes the surface area is dramatically more.
Still, I want to play with temps and flow rates on mine and see if I can overcome the surface area differences.
I think this has convinced me to go with glycol over a fridge for purely the reason that it sounds like fun to solve this problem
and now....you are so far around the bend nobody can see you. Except me.
Heat capacity indicates the stored energy of the system, but is less useful in equilibrium conditions, since for the ambient air, it is essentially an infinite reservoir for all intents and purposes (you aren’t gonna cool your garage appreciably with your conical). If you insulated in a box, then you could use cp to understand how long it would take you to reach equilibrium (lower the temp of the insulated box).
As to the assumption that neoprenecan be neglected for conical area, it cannot, since its R-value is quite low compared with closed cell foam or other materials used commonly for insulation. It is a speed bump for the heat rather than a blocker.
For reference, I have a jacketed (large cooling surface area) internally insulated (high R value, though I haven’t measured it) conical that keeps beer at 30 with glycol at 28 in a garage that’s 90+...
Thanks for the education!
Am I understanding correctly that the neoprene jacket is really of no value?
Those are great numbers you are achieving in a 90F+ garage. The jacketed with the large cooling surface apparently makes all the difference. I have been looking at jacketed concicals the past couple of weeks or so. If you don't mind me asking, who manufactures your jacket conical? Is it correct to assume you are happy with it?
The neoprene is not useless (still has a higher R value than 0 of the metal), but somewhat like a wetsuit as opposed to foam, so it’s a little more form than function in terms of looks. If you want to calculate the R value, put a thermometer inside and another one outside In the same location and you can directly measure its effectiveness. If you use neoprene conductivity estimates and thickness you also have a poor man’s heat flux gauge!
I have a Stout 20G, which is well engineered and built with all the ports you could wish for - you can see a pic in Ss Brewtech 1/5 hp glycol chiller
If/when I look to buy another one, I would also look at Brewer’s hardware, as these weren’t around when I bought the Stout. If I remember, they offer in smaller sizes and are less expensive (though still more than a spike/SS Unitank due to higher material and labor cost), but I haven’t done a work up on functionality and build quality comparison. All told, if you can make a coiled system work, go for it, as that’s the best bang for the buck, but those are engineered on the edge for low cost, so aren’t robust to meet everyone’s application conditions.
The only issue I’ve had with the Stout was initial sealing on the top gasket, but a round of leak detector combined with torquing the eye bolts and that was addressed. I don’t remove the lid and CIP through the 4 inch port on top (the SS low flow CIP so can use it with one of my old SS March pumps), so no usability issue for me. Others like to get in and hand clean, but I’d rather not mar the mirror-like finish on the inside of the stout, so let the PBW and CIP do the scratch-free work.
The best control volume to draw is around the beer to capture the heat rate in and out. The bit that the cited equations are missing is that they are thermodynamic equations rather than heat transfer equations. They can be manipulated to q=h A deltaT, where h is the convective heat transfer coefficient, A is the surface area, and delta T is the temp gradient. Using the beer as the CV and solving the equilibrium state gives
h_ambient A_ambient (T_amb - T_beer) = h_coil A_coil (T_beer - T_coil).
I just had another though for you @mongoose33.... Also now that I'm joining this crusade I found that old necro thread of yours with your temp data.
Your coil in temp is 28 and your coil out temp is 35, meaning you have a temperature gradient of about 7 degrees between the top of your coil and the bottom of your coil sitting in the beer. That means that the full surface area of your coil is not pulling heat at the maximum possible rate because you have a different deltaT between the beer and the coil at the top vs the coil at the bottom.
if you did use a bigger pump and pushed your glycol so fast through your coil, that your return temp is say, 30, you would have a much larger surface area of coil at a colder temperature working for you that might help overcome the heat gain from the ambient.
We also have a couple things working in our favor.
The wall thickness of the coil is much thinner than the wall thickness of the fermenter, which improves heat transfer, eating into the surface area deficit.
The convective heat transfer coefficient of a fluid is directly affected by its speed of movement. So if the air is not moving around the fermenter, but the beer inside the fermenter is mixed/stirred it will preferentially affect the chilling ability over the heat loss, especially given that the glycol inside the coil is moving also. This is where the moving blanket would really shine I think, as it restricts air movement.
If you can find me that pump with greater capacity at a price point reasonably similar to the others, and that will fit in my penguin, I'd be willing to try it.
Betcha can't.
This pump should work, with a draw tube in the glycol, and you could even insulate the head a little. Just move your inkbird glycol probe to the outlet of the pump, and keep the setpoint at 28. The glycol reservoir will cool down low enough to keep the temperature at the pump outlet at the same temperature as always.
Setting aside the pump being outside, in ambient, it's rated at 320 gph. I'm doing 30 gph. What will a tenfold increase in throughput (and pressure?) do to the system? And that return stream--will it splash back into the reservoir of the Penguin?
You guys should start a glycol chiller thread! (Just saying...)
I use mini-sight glasses above the butterfly valves on both my conicals. They are 1" bore and approximately 2 1/2" long. Since they are above the valve, I can see when the trub (which settles before the yeast) has all been dumped. Then I remove that jar, santize the valve with a spritz of Star San, and collect clean yeast in a new jar. I can also see when the yeast ends and the beer begins. Works great, gives visual cues of fermentation, minimizes wasted beer, recovers huge amounts of clean yeast, and only adds less than 3" to the overall length of the dump port.I've often wanted to attach my sightglass to the bottom TC port, before the Butterfly and elbow, but I dont have that much room underneath the conical. I'd need leg extensions for that which would make my half barrel fermenter too tall for the upright freezer.
I could probably do that though with leg extensions on the CF5; and get that brace along with it...
I use mini-sight glasses above the butterfly valves on both my conicals. They are 1" bore and approximately 2 1/2" long. Since they are above the valve, I can see when the trub (which settles before the yeast) has all been dumped. Then I remove that jar, santize the valve with a spritz of Star San, and collect clean yeast in a new jar. I can also see when the yeast ends and the beer begins. Works great, gives visual cues of fermentation, minimizes wasted beer, recovers huge amounts of clean yeast, and only adds less than 3" to the overall length of the dump port.
Brooo Brother
Nobody, as you wouldn't want to agitate wort/beer in the fermenter at any point in time.Who wants to build a physical model or FEA and find out?
That sounds like a pretty good system - can you post a picture? Or maybe a link to the sight glass you're using?
That sounds like a pretty good system - can you post a picture? Or maybe a link to the sight glass you're using?