Martin,
I suggest you read the Kolbach paper (
http://www.wetnewf.org/pdfs/Brewing_articles/KolbachPaper.pdf) again paying special attention to paragraphs like this one:
"From experience there are insufficient experimental results for us to be able to indicate to
at least some extent precisely how much the pH of the wort changes when the residual
alkalinity changes by a particular amount."
Indeed within the paper he makes the statements
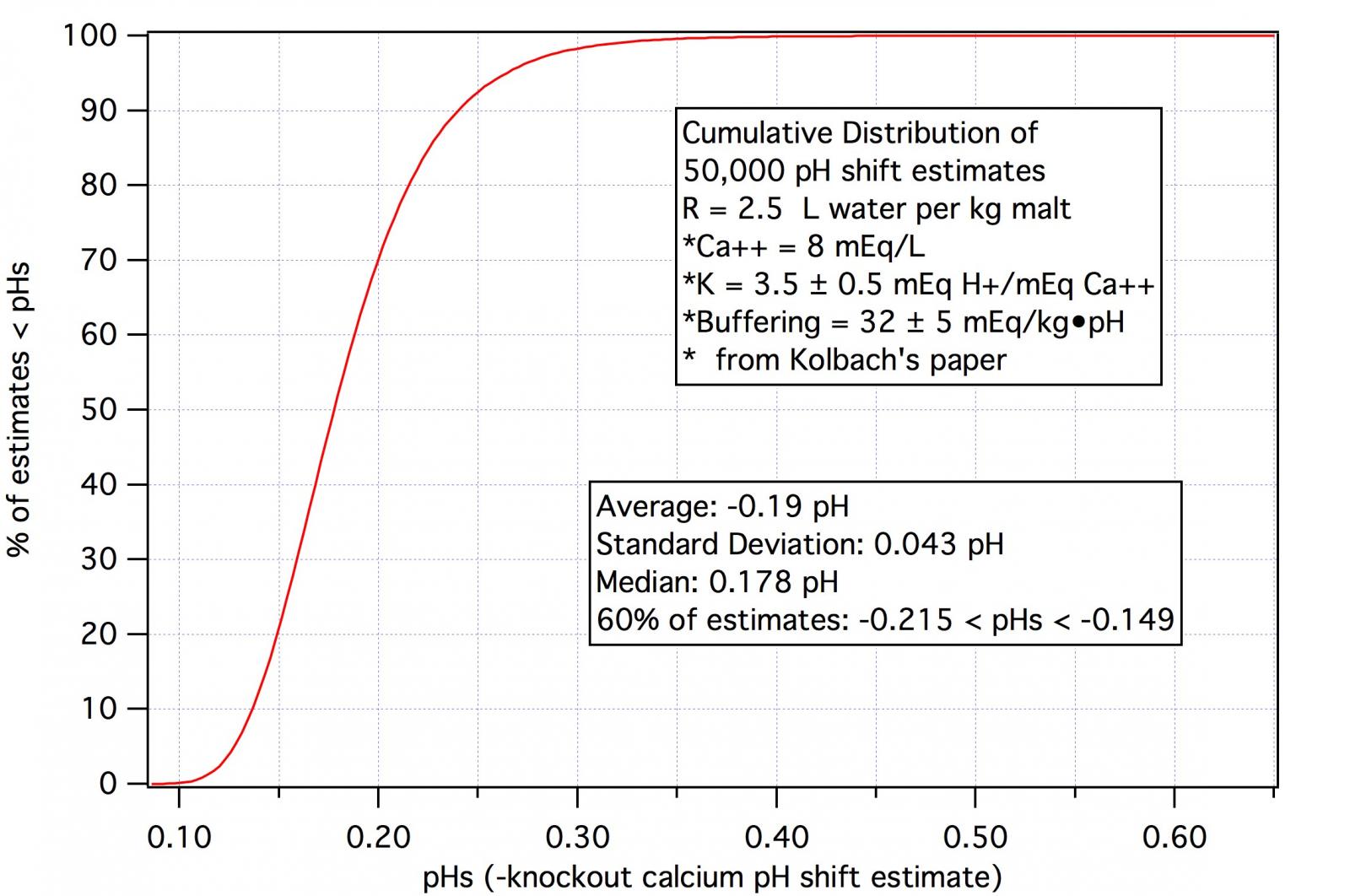
1) 3.5 mEq calcium offset 1 mEq alkalinity
2) A 10 ° dH (3.574 mEq/L) shifts the pH of a 12 °P beer by 0.3 pH
3) The buffering of wort is 32 mEq/ig•pH
Statements 1 and 3 imply a shift of 0.279 pH from a 10 ° RA shift which while not terribly different from 0.3 is different nevertheless. Furthermore, measurements on modern malts suggest that wort bufferings are going to be more like 50 mEq/kg•pH and, of course, will vary with the water to grist ratio i.e. the strength of the beer.
All of this suggests that there is going to be a fair amount of variability, on a percentage basis at least, in the pH shift per unit of RA shift over the range of typical brewing conditions. But the absolute values of shift are low. It is only in exceptional cases (and we note that Kolbach chose super hard Dortmund water for his examples) that the pH shifts are greater than 0.1.
I have not done any formal experimental evaluations of the shift in part because I know that the considerations mentioned above would mean that such a project would be no mean undertaking. If Kolbach couldn't do it I don't see how I could. I have, therefore, cleaved to the 3.5 mEq of calcium number and use that as the basis for all calculations. I think I have good estimates of malt buffering capacities so that part of my protocol is fairly robust but I am well aware that the actual number I would encounter with any real grist will not be 3.5 but only near it. Then there is the issue of knockout vs runoff wort. Even with all this uncertainty varying assumptions typically make differences in estimated pH shifts of a couple of hundredths. Even with the obscenely hard Dortmund water of Kolbach's paper (8 mEq/L or 400 ppm after decarbonation) the difference between 0.3 pH per 10 ° and using 3.5 mEq calcium to cancel 1 mEq of alkalinity with sound values for malt buffering the estimates are, respectively -0.2 pH and -0.1 pH at knockout and, presumably -0.1 and -0.5 in the mash tun. For more usual hardnesses the differences are a couple of hundredths.








































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)