pensphreak
Well-Known Member
I put in (what I think) are the same values for an upcoming Oktoberfest that I'm brewing and the output from both of these spreadsheets is pretty different (5.49 vs 5.2 pH). Can someone take a look at my spreadsheets and tell me what I'm doing wrong?
Background: I'm brewing with 100% RO water and normally I just follow the primer (1g CaCL2/gal of water). I batch sparge with (in this case) 4.88gal of mash water and 5.69gal of sparge water.
Here's my recipe: http://pensphreak.com/homebrew/Oktoberfest%202.0.html
Here's the 2 spreadsheets: Bru'n Water and EZ Water
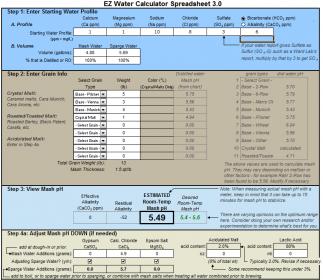
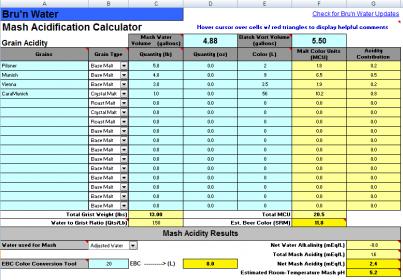
Background: I'm brewing with 100% RO water and normally I just follow the primer (1g CaCL2/gal of water). I batch sparge with (in this case) 4.88gal of mash water and 5.69gal of sparge water.
Code:
Amt Name
5.00 g Calcium Chloride
5 lbs Pilsner (2 Row) Ger (2.0 SRM)
4 lbs Munich Malt (9.0 SRM)
3 lbs Vienna Malt (3.5 SRM)
1 lbs Caramunich Malt (56.0 SRM)Here's my recipe: http://pensphreak.com/homebrew/Oktoberfest%202.0.html
Here's the 2 spreadsheets: Bru'n Water and EZ Water









































