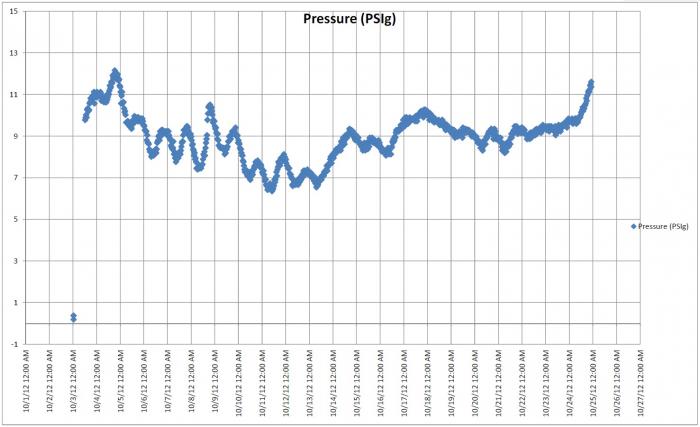
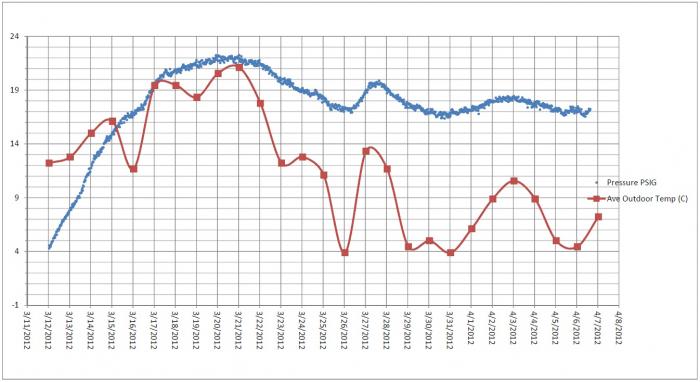
My thought is that it will tell you that the yeast was active, not that it is "still" active. Also, the gauge should give you an idea when the beer is done carbonating. After peaking, the reading should drop. When this dropping stops and stabilizes the beer should be fully carbed. No?
Yes, active as in present and alive. Yep, I would say that when the pressure stops dropping the beer should be fully carbed which should take about 2-3 weeks under normal circumstances. I've never had a beer fail to carbonate in the bottle and only rarely has any been fully carbonated in less than two weeks. Again, putting a gauge on a bottle would be interesting to observe, but that's about all it would be for me.































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)